Impact of LDL apheresis on atheroprotective reverse cholesterol transport pathway in familial hypercholesterolemia
- PMID: 22338009
- PMCID: PMC3307653
- DOI: 10.1194/jlr.M024141
Impact of LDL apheresis on atheroprotective reverse cholesterol transport pathway in familial hypercholesterolemia
Abstract
In familial hypercholesterolemia (FH), low HDL cholesterol (HDL-C) levels are associated with functional alterations of HDL particles that reduce their capacity to mediate the reverse cholesterol transport (RCT) pathway. The objective of this study was to evaluate the consequences of LDL apheresis on the efficacy of the RCT pathway in FH patients. LDL apheresis markedly reduced abnormal accelerated cholesteryl ester transfer protein (CETP)-mediated cholesteryl ester (CE) transfer from HDL to LDL, thus reducing their CE content. Equally, we observed a major decrease (-53%; P < 0.0001) in pre-β1-HDL levels. The capacity of whole plasma to mediate free cholesterol efflux from human macrophages was reduced (-15%; P < 0.02) following LDL apheresis. Such reduction resulted from a marked decrease in the ABCA1-dependent efflux (-71%; P < 0.0001) in the scavenger receptor class B type I-dependent efflux (-21%; P < 0.0001) and in the ABCG1-dependent pathway (-15%; P < 0.04). However, HDL particles isolated from FH patients before and after LDL apheresis displayed a similar capacity to mediate cellular free cholesterol efflux or to deliver CE to hepatic cells. We demonstrate that rapid removal of circulating lipoprotein particles by LDL apheresis transitorily reduces RCT. However, LDL apheresis is without impact on the intrinsic ability of HDL particles to promote either cellular free cholesterol efflux from macrophages or to deliver CE to hepatic cells.
Figures
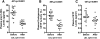
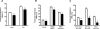
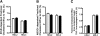

References
-
- Jansen A. C., van Wissen S., Defesche J. C., Kastelein J. J. 2002. Phenotypic variability in familial hypercholesterolaemia: an update. Curr. Opin. Lipidol. 13: 165–171 - PubMed
-
- Jansen A. C., van Aalst-Cohen E. S., Tanck M. W., Trip M. D., Lansberg P. J., Liem A. H., van Lennep H. W., Sijbrands E. J., Kastelein J. J. 2004. The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolaemia: data in 2400 patients. J. Intern. Med. 256: 482–490 - PubMed
-
- van Aalst-Cohen E. S., Jansen A. C., Boekholdt S. M., Tanck M. W., Fontecha M. R., Cheng S., Li J., Defesche J. C., Kuivenhoven J. A., Kastelein J. J. 2005. Genetic determinants of plasma HDL-cholesterol levels in familial hypercholesterolemia. Eur. J. Hum. Genet. 13: 1137–1142 - PubMed
-
- Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R., Jr, Bangdiwala S., Tyroler H. A. 1989. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 79: 8–15 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

