Developmental regulation of membrane excitability in rat spinal lamina I projection neurons
- PMID: 22338021
- PMCID: PMC3362289
- DOI: 10.1152/jn.00899.2011
Developmental regulation of membrane excitability in rat spinal lamina I projection neurons
Abstract
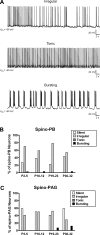
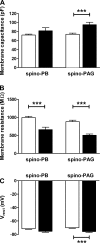
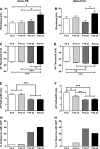
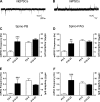
It is now universally recognized that neonates can experience considerable pain. While spinal lamina I neurons projecting to the brain contribute to the generation of hyperalgesia, nothing is known about their electrophysiological properties during early life. Here we have used in vitro whole cell patch-clamp recordings in rat spinal cord slices to determine whether the intrinsic membrane properties of lamina I projection neurons, as well as their synaptic inputs, are developmentally regulated during the early postnatal period. Projection neurons were identified via retrograde transport of DiI injected into the parabrachial nucleus (PB) or periaqueductal gray (PAG) and characterized at postnatal days (P)2-5, P10-12, P19-23, and P30-32. Both spino-PB and spino-PAG neurons demonstrated an age-dependent reduction in spike threshold and duration at room temperature, which was accompanied by a developmental increase in the frequency of miniature excitatory and inhibitory postsynaptic currents. Notably, in both groups, age-dependent changes in the passive membrane properties or rheobase only occurred after the third postnatal week. However, spontaneous activity was significantly more prevalent within the developing spino-PB population and was dominated by an irregular pattern of discharge. In addition, while the instantaneous firing frequency remained unaltered in spino-PB neurons during the first weeks of life, spino-PAG cells fired at a higher rate at P19-23 compared with younger groups, suggesting that the gain of parallel ascending nociceptive pathways may be independently regulated during development. Overall, these results demonstrate that intrinsic membrane excitability is modulated in a cell type-specific manner within developing spinal nociceptive circuits.
Figures



Similar articles
-
Inward-rectifying K+ (Kir2) leak conductance dampens the excitability of lamina I projection neurons in the neonatal rat.Neuroscience. 2016 Dec 17;339:502-510. doi: 10.1016/j.neuroscience.2016.10.027. Epub 2016 Oct 14. Neuroscience. 2016. PMID: 27751963 Free PMC article.
-
Synaptic input of rat spinal lamina I projection and unidentified neurones in vitro.J Physiol. 2005 Jul 15;566(Pt 2):355-68. doi: 10.1113/jphysiol.2005.088567. Epub 2005 May 5. J Physiol. 2005. PMID: 15878938 Free PMC article.
-
Distinctive membrane and discharge properties of rat spinal lamina I projection neurones in vitro.J Physiol. 2004 Mar 1;555(Pt 2):527-43. doi: 10.1113/jphysiol.2003.054049. Epub 2003 Dec 23. J Physiol. 2004. PMID: 14694142 Free PMC article.
-
Intrinsic and synaptic properties of adult mouse spinoperiaqueductal gray neurons and the influence of neonatal tissue damage.Pain. 2023 Apr 1;164(4):905-917. doi: 10.1097/j.pain.0000000000002787. Epub 2022 Sep 23. Pain. 2023. PMID: 36149785 Free PMC article.
-
Homologies of spinal ascending nociceptive pathways between rats and macaques: can we transpose to human? A review and analysis of the literature.Surg Radiol Anat. 2023 Nov;45(11):1443-1460. doi: 10.1007/s00276-023-03212-w. Epub 2023 Jul 28. Surg Radiol Anat. 2023. PMID: 37507602 Review.
Cited by
-
[Changes of membrane properties and synaptic stability of rat retinal ganglion cells during postnatal development].Nan Fang Yi Ke Da Xue Xue Bao. 2018 Aug 30;38(9):1100-1106. doi: 10.12122/j.issn.1673-4254.2018.09.13. Nan Fang Yi Ke Da Xue Xue Bao. 2018. PMID: 30377110 Free PMC article. Chinese.
-
An electrophysiologist's guide to dorsal horn excitability and pain.Front Cell Neurosci. 2025 Apr 2;19:1548252. doi: 10.3389/fncel.2025.1548252. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40241846 Free PMC article. Review.
-
Connectivity of pacemaker neurons in the neonatal rat superficial dorsal horn.J Comp Neurol. 2015 May 1;523(7):1038-1053. doi: 10.1002/cne.23706. Epub 2015 Feb 17. J Comp Neurol. 2015. PMID: 25380417 Free PMC article.
-
Inward-rectifying K+ (Kir2) leak conductance dampens the excitability of lamina I projection neurons in the neonatal rat.Neuroscience. 2016 Dec 17;339:502-510. doi: 10.1016/j.neuroscience.2016.10.027. Epub 2016 Oct 14. Neuroscience. 2016. PMID: 27751963 Free PMC article.
-
Neonatal tissue injury reduces the intrinsic excitability of adult mouse superficial dorsal horn neurons.Neuroscience. 2014 Jan 3;256:392-402. doi: 10.1016/j.neuroscience.2013.10.057. Epub 2013 Nov 1. Neuroscience. 2014. PMID: 24184978 Free PMC article.
References
-
- Altman J, Bayer SA. Atlas of Prenatal Rat Brain Development. Boca Raton, FL: CRC, 1995
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

