Effects of high-fat diet feeding on Znt8-null mice: differences between β-cell and global knockout of Znt8
- PMID: 22338079
- PMCID: PMC3774340
- DOI: 10.1152/ajpendo.00448.2011
Effects of high-fat diet feeding on Znt8-null mice: differences between β-cell and global knockout of Znt8
Abstract
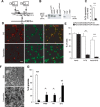
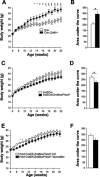
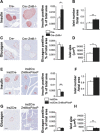
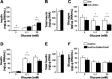
Genomewide association studies have linked a polymorphism in the zinc transporter 8 (Znt8) gene to higher risk of developing type 2 diabetes. Znt8 is highly expressed in pancreatic β-cells where it is involved in the regulation of zinc transport into granules. However, Znt8 is also expressed in other tissues including α-cells, where its function is as yet unknown. Previous work demonstrated that mice lacking Znt8 globally were more susceptible to diet-induced obesity (Lemaire et al., Proc Natl Acad Sci USA 106: 14872-14877, 2009; Nicolson et al., Diabetes 58: 2070-2083, 2009). Therefore, the main goal of this study was to examine the physiological impact of β-cell-specific Znt8 deficiency in mice during high-fat high-calorie (HFHC) diet feeding. For these studies, we used β-cell-specific Znt8 knockout (Ins2Cre:Znt8loxP/loxP) and whole body Znt8 knockout (Cre-:Znt8(-/-)) mice placed on a HFHC diet for 16 wk. Ins2Cre:Znt8loxP/loxP mice on HFHC diet had similar body weights throughout the study but displayed impaired insulin biosynthesis and secretion and were glucose intolerant compared with littermate control Ins2Cre mice. In contrast, Cre-:Znt8(-/-) mice became remarkably obese, hyperglycemic, hyperinsulinemic, insulin resistant, and glucose intolerant compared with littermate control Cre- mice. These data show that β-cell Znt8 alone does not considerably aggravate weight gain and glucose intolerance during metabolic stress imposed by an HFHC diet. However, global loss of Znt8 is involved in exacerbating diet-induced obesity and resulting insulin resistance, and this may be due to the loss of Znt8 activity in a tissue other than the β-cell. Thus, our data suggest that Znt8 contributes to the risk of developing type 2 diabetes through β-cell- and non-β-cell-specific effects.
Figures





References
-
- Alemzadeh R, Holshouser S, Massey P, Koontz J. Chronic suppression of insulin by diazoxide alters the activities of key enzymes regulating hepatic gluconeogenesis in Zucker rats. Eur J Endocrinol 146: 871–879, 2002 - PubMed
-
- Alemzadeh R, Jacobs W, Pitukcheewanont P. Antiobesity effect of diazoxide in obese Zucker rats. Metabolism 45: 334–341, 1996 - PubMed
-
- Alemzadeh R, Langley G, Upchurch L, Smith P, Slonim AE. Beneficial effect of diazoxide in obese hyperinsulinemic adults. J Clin Endocrinol Metab 83: 1911–1915, 1998 - PubMed
-
- Alemzadeh R, Slonim AE, Zdanowicz MM, Maturo J. Modification of insulin resistance by diazoxide in obese Zucker rats. Endocrinology 133: 705–712, 1993 - PubMed
-
- Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M, Fujino MA, Niijima A, Meguid MM, Kasuga M. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology 124: 1325–1336, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

