Exploiting metabolic differences in glioma therapy
- PMID: 22339075
- PMCID: PMC3638785
- DOI: 10.2174/157016312803305906
Exploiting metabolic differences in glioma therapy
Abstract
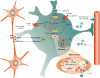
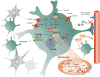
Brain function depends upon complex metabolic interactions amongst only a few different cell types, with astrocytes providing critical support for neurons. Astrocyte functions include buffering the extracellular space, providing substrates to neurons, interchanging glutamate and glutamine for synaptic transmission with neurons, and facilitating access to blood vessels. Whereas neurons possess highly oxidative metabolism and easily succumb to ischemia, astrocytes rely more on glycolysis and metabolism associated with synthesis of critical intermediates, hence are less susceptible to lack of oxygen. Astrocytoma and higher grade glioma cells demonstrate both basic metabolic mechanisms of astrocytes as well as tumors in general, e.g. they show a high glycolytic rate, lactate extrusion, ability to proliferate even under hypoxia, and opportunistic use of mechanisms to enhance metabolism and blood vessel generation, and suppression of cell death pathways. There may be differences in metabolism between neurons, normal astrocytes and astrocytoma cells, providing therapeutic opportunities against astrocytomas, including a wide range of enzyme and transporter differences, regulation of hypoxia-inducible factor (HIF), glutamate uptake transporters and glutamine utilization, differential sensitivities of monocarboxylate transporters, presence of glycogen, high interlinking with gap junctions, use of NADPH for lipid synthesis, utilizing differential regulation of synthetic enzymes (e.g. isocitrate dehydrogenase, pyruvate carboxylase, pyruvate dehydrogenase, lactate dehydrogenase, malate-aspartate NADH shuttle) and different glucose uptake mechanisms. These unique metabolic susceptibilities may augment conventional therapeutic attacks based on cell division differences and surface receptors alone, and are starting to be implemented in clinical trials.
Conflict of interest statement
Figures
References
-
- Escartin C, Valette J, Lebon V, et al. Neuron-astrocyte interactions in the regulation of brain energy metabolism: a focus on NMR spectroscopy. J Neurochem. 2006;99(2):393–401. - PubMed
-
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27(2):219–49. - PubMed
-
- Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res. 1999;24(2):321–9. - PubMed
-
- Verkhratsky A, Toescu EC. Neuronal-glial networks as substrate for CNS integration. J Cell Mol Med. 2006;10(4):826–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

