NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns
- PMID: 22340513
- PMCID: PMC6837626
- DOI: 10.1179/1351000212Y.0000000001
NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns
Abstract
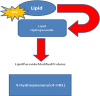
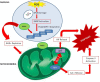
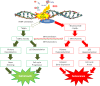
In the twentieth century, NAD+ research generated multiple discoveries. Identification of the important role of NAD+ as a cofactor in cellular respiration and energy production was followed by discoveries of numerous NAD+ biosynthesis pathways. In recent years, NAD+ has been shown to play a unique role in DNA repair and protein deacetylation. As discussed in this review, there are close interactions between oxidative stress and immune activation, energy metabolism, and cell viability in neurodegenerative disorders and ageing. Profound interactions with regard to oxidative stress and NAD+ have been highlighted in the present work. This review emphasizes the pivotal role of NAD+ in the regulation of DNA repair, stress resistance, and cell death, suggesting that NAD+ synthesis through the kynurenine pathway and/or salvage pathway is an attractive target for therapeutic intervention in age-associated degenerative disorders. NAD+ precursors have been shown to slow down ageing and extend lifespan in yeasts, and protect severed axons from degeneration in animal models neurodegenerative diseases.
Figures




References
-
- Elvehjem C, Madden R, Strong F, Woolley D. Relation of nicotinic acid and nicotinic acid amide to canine black tongue. J Am Chem Soc 1937;59:1767–8.
-
- Elvehjem C. Pellagra – a deficiency disease. Proc Am Philos Soc 1949;93:335–9. - PubMed
-
- Broer S, JA C, Rasko J. Neutral amino acid transport in epithelial cells and its malfunction in Hartnup disorder. Biochem Soc Trans 2005;33:233–6. - PubMed
-
- Monteiro J, da Cunha D, Filho D, SilvaVergara M, dos Santos V, da Costas J , et al.. Niacin metabolite excretion in alcholic pellagra and AIDS patients with and without diarrhea. Nutrition 2004;20:778–82. - PubMed
-
- Comaish J, Felix R, McGrath H. Topically applied niacinamide in ioniazid-induced pellagra. Arch Dermatol 1976;112:70–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
