Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial
- PMID: 22340966
- PMCID: PMC3314999
- DOI: 10.1016/S1470-2045(11)70364-3
Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial
Abstract
Background: Hot flushes and night sweats (HFNS) affect 65-85% of women after breast cancer treatment; they are distressing, causing sleep problems and decreased quality of life. Hormone replacement therapy is often either undesirable or contraindicated. Safe, effective non-hormonal treatments are needed. We investigated whether cognitive behavioural therapy (CBT) can help breast cancer survivors to effectively manage HFNS.
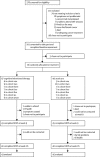
Methods: In this randomised controlled trial, we recruited women from breast clinics in London, UK, who had problematic HFNS (minimum ten problematic episodes a week) after breast-cancer treatment. Participants were randomly allocated to receive either usual care or usual care plus group CBT (1:1). Randomisation was done in blocks of 12-20 participants, stratifying by age (younger than 50 years, 50 years or older), and was done with a computer-generated sequence. The trial statistician and researchers collecting outcome measures were masked to group allocation. Group CBT comprised one 90 min session a week for 6 weeks, and included psycho-education, paced breathing, and cognitive and behavioural strategies to manage HFNS. Assessments were done at baseline, 9 weeks, and 26 weeks after randomisation. The primary outcome was the adjusted mean difference in HFNS problem rating (1-10) between CBT and usual care groups at 9 weeks after randomisation. Analysis of the primary endpoint was done by modified intention to treat. The trial is registered, ISRCTN13771934, and was closed March 15, 2011.
Findings: Between May 5, 2009, and Aug 27, 2010, 96 women were randomly allocated to group CBT (n=47) or usual care (n=49). Group CBT significantly reduced HFNS problem rating at 9 weeks after randomisation compared with usual care (mean difference -1·67, 95% CI -2·43 to -0·91; p<0·0001) and improvements were maintained at 26 weeks (mean difference -1·76, -2·54 to -0·99; p<0·0001). We recorded no CBT-related adverse events.
Interpretation: Group CBT seems to be a safe and effective treatment for women who have problematic HFNS after breast cancer treatment with additional benefits to mood, sleep, and quality of life. The treatment could be incorporated into breast cancer survivorship programmes and delivered by trained breast cancer nurses.
Funding: Cancer Research UK.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Mind over menopausal symptoms in women with breast cancer.Lancet Oncol. 2012 Mar;13(3):227-9. doi: 10.1016/S1470-2045(11)70381-3. Epub 2012 Feb 15. Lancet Oncol. 2012. PMID: 22340964 No abstract available.
-
Breast cancer: Minding your hot flashes.Nat Rev Clin Oncol. 2012 Mar 13;9(4):189. doi: 10.1038/nrclinonc.2012.30. Nat Rev Clin Oncol. 2012. PMID: 22411343 No abstract available.
-
Placebo effect in hot flush research.Lancet Oncol. 2012 May;13(5):e188; author reply e190. doi: 10.1016/S1470-2045(12)70197-3. Lancet Oncol. 2012. PMID: 22554544 No abstract available.
References
-
- Office for National Statistics . Cancer Statistics registrations: registrations of cancer diagnosed in 2008, England. National Statistics; London: 2010.
-
- Carpenter J, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29:16–25. - PubMed
-
- Hunter MS, Grunfeld EA, Mittal S. Menopausal symptoms in women with breast cancer: prevalence and treatment preferences. Psychooncology. 2004;13:769–778. - PubMed
-
- Gupta P, Sturdee D, Palin S. Menopausal symptoms in women treated for breast cancer: the prevalence and severity of symptoms and their perceived effects on quality of life. Climacteric. 2006;9:49–58. - PubMed
-
- Howell A, Cuzick J, Baum M. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

