5-year continence rates, satisfaction and adverse events of burch urethropexy and fascial sling surgery for urinary incontinence
- PMID: 22341290
- PMCID: PMC3586411
- DOI: 10.1016/j.juro.2011.11.087
5-year continence rates, satisfaction and adverse events of burch urethropexy and fascial sling surgery for urinary incontinence
Abstract
Purpose: We characterized continence, satisfaction and adverse events in women at least 5 years after Burch urethropexy or fascial sling with longitudinal followup of randomized clinical trial participants.
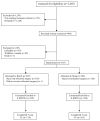
Materials and methods: Of 655 women who participated in a randomized surgical trial comparing the efficacy of the Burch and sling treatments 482 (73.6%) enrolled in this long-term observational study. Urinary continence status was assessed yearly for a minimum of 5 years postoperatively. Continence was defined as no urinary leakage on a 3-day voiding diary, and no self-reported stress incontinence symptoms and no stress incontinence surgical re-treatment.
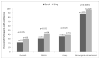
Results: Incontinent participants were more likely to enroll in the followup study than continent patients (85.5% vs 52.2%) regardless of surgical group (p<0.0001). Overall the continence rates were lower in the Burch urethropexy group than in the fascial sling group (p=0.002). The continence rates at 5 years were 24.1% (95% CI 18.5 to 29.7) vs 30.8% (95% CI 24.7 to 36.9), respectively. Satisfaction at 5 years was related to continence status and was higher in women undergoing sling surgery (83% vs 73%, p=0.04). Satisfaction decreased with time (p=0.001) and remained higher in the sling group (p=0.03). The 2 groups had similar adverse event rates (Burch 10% vs sling 9%) and similar numbers of participants with adverse events (Burch 23 vs sling 22).
Conclusions: Continence rates in both groups decreased substantially during 5 years, yet most women reported satisfaction with their continence status. Satisfaction was higher in continent women and in those who underwent fascial sling surgery, despite the voiding dysfunction associated with this procedure.
Trial registration: ClinicalTrials.gov NCT00064662.
Copyright © 2012 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Rehman H, Bezerra CBC, Bruschini H, et al. Traditional suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2011;1 - PubMed
-
- Sand PK, Winkler H, Blackhurst DW, et al. A prospective randomized study comparing modified Burch retropubic urethropexy and suburethral sling for treatment of genuine stress incontinence with low-pressure urethra. Am J Obstet Gynecol. 2000;182:30–34. - PubMed
-
- Novara G, Galfano A, Boscolo-Berto R, et al. Complication rates of tension-free midurethral slings in the treatment of female stress urinary incontinence: a systematic review and meta-analysis of randomized controlled trials comparing tension-free midurethral tapes to other surgical procedures and different devices. Eur Urol. 2008;53:288–308. - PubMed
-
- Tennstedt S Urinary Incontinence Treatment N. Design of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) Urol. 2005;66:1213–1217. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U01 DK60393/DK/NIDDK NIH HHS/United States
- U01 DK060379/DK/NIDDK NIH HHS/United States
- U01 DK058234/DK/NIDDK NIH HHS/United States
- U01 DK58234/DK/NIDDK NIH HHS/United States
- U01 DK060393/DK/NIDDK NIH HHS/United States
- U01 DK60379/DK/NIDDK NIH HHS/United States
- U01 DK060380/DK/NIDDK NIH HHS/United States
- U01 DK060397/DK/NIDDK NIH HHS/United States
- U01 DK60380/DK/NIDDK NIH HHS/United States
- U01 DK60395/DK/NIDDK NIH HHS/United States
- U01 DK060395/DK/NIDDK NIH HHS/United States
- U01 DK58229/DK/NIDDK NIH HHS/United States
- U01 DK60397/DK/NIDDK NIH HHS/United States
- U01 DK58231/DK/NIDDK NIH HHS/United States
- 60401/PHS HHS/United States
- U01 DK058229/DK/NIDDK NIH HHS/United States
- U01 DK58225/DK/NIDDK NIH HHS/United States
- U01 DK058225/DK/NIDDK NIH HHS/United States
- U01 DK058231/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

