Aberrant amplification of the crosstalk between canonical Wnt signaling and N-glycosylation gene DPAGT1 promotes oral cancer
- PMID: 22341307
- PMCID: PMC3362683
- DOI: 10.1016/j.oraloncology.2012.01.010
Aberrant amplification of the crosstalk between canonical Wnt signaling and N-glycosylation gene DPAGT1 promotes oral cancer
Abstract
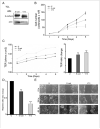
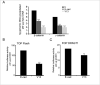
Oral cancer is one of the most aggressive epithelial malignancies, whose incidence is on the rise. Previous studies have shown that in a subset of human oral squamous cell carcinoma (OSCC) tumor specimens, overexpression of the DPAGT1 gene, encoding the dolichol-P-dependent N-acetylglucoseamine-1-phosphate transferase, a key regulator of the metabolic pathway of protein N-glycosylation, drives tumor cell discohesion by inhibiting E-cadherin adhesive function. Recently, we reported that DPAGT1 was a target of the canonical Wnt signaling pathway. Here, we link overexpression of DPAGT1 in human OSCC tumor specimens to aberrant activation of canonical Wnt signaling. We report dramatic increases in β- and γ-catenins at the DPAGT1 promoter and correlate them with reduced expression of a Wnt inhibitor, Dickkopf-1 (Dkk-1). Using human squamous carcinoma cell lines of the head and neck, we show that partial inhibition of DPAGT1 reduces canonical Wnt signaling, indicating that DPAGT1 and canonical Wnt signaling function in a positive feedback loop. We provide evidence that E-cadherin inhibits DPAGT1, canonical Wnt signaling and the OSCC cancer phenotype by depleting nuclear β- and γ-catenins, with hypoglycosylated E-cadherin being the most effective. This suggests that in human OSCC, extensive N-glycosylation of E-cadherin compromises its ability to inhibit canonical Wnt signaling and DPAGT1 expression. Our studies reveal a novel interplay between DPAGT1/N-glycosylation and canonical Wnt signaling and suggest that dysregulation of this crosstalk is a key mechanism underlying OSCC. They also suggest that partial inhibition of DPAGT1 may represent an effective way to restore normal interactions among these essential pathways in oral cancer.
Published by Elsevier Ltd.
Figures





References
-
- Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res. 2008;87:14–32. - PubMed
-
- Zuo J-H, Zhu W, Li M-Y, Li X-H, Yi H, Zeng G-Q, et al. Activation of EGFR Promotes Squamous Carcinoma SCC10A Cell Migration and Invasion via Inducins EMT-like PhenotypeCHange and MMP-9-Mediated Degradation of E-cadherin. J Cell Biochem. 2011;112:2508–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

