Spatial organization of the mouse genome and its role in recurrent chromosomal translocations
- PMID: 22341456
- PMCID: PMC3320767
- DOI: 10.1016/j.cell.2012.02.002
Spatial organization of the mouse genome and its role in recurrent chromosomal translocations
Abstract
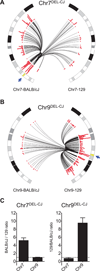
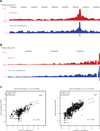
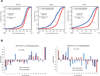
The extent to which the three-dimensional organization of the genome contributes to chromosomal translocations is an important question in cancer genomics. We generated a high-resolution Hi-C spatial organization map of the G1-arrested mouse pro-B cell genome and used high-throughput genome-wide translocation sequencing to map translocations from target DNA double-strand breaks (DSBs) within it. RAG endonuclease-cleaved antigen-receptor loci are dominant translocation partners for target DSBs regardless of genomic position, reflecting high-frequency DSBs at these loci and their colocalization in a fraction of cells. To directly assess spatial proximity contributions, we normalized genomic DSBs via ionizing radiation. Under these conditions, translocations were highly enriched in cis along single chromosomes containing target DSBs and within other chromosomes and subchromosomal domains in a manner directly related to pre-existing spatial proximity. By combining two high-throughput genomic methods in a genetically tractable system, we provide a new lens for viewing cancer genomes.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Barreca A, Lasorsa E, Riera L, Machiorlatti R, Piva R, Ponzoni M, Kwee I, Bertoni F, Piccaluga PP, Pileri SA, et al. Anaplastic lymphoma kinase in human cancer. Journal of molecular endocrinology. 2011;47:R11–R23. - PubMed
-
- Boyle S, Gilchrist S, Bridger JM, Mahy NL, Ellis JA, Bickmore WA. The spatial organization of human chromosomes within the nuclei of normal and emerin-mutant cells. Human molecular genetics. 2001;10:211–219. - PubMed
-
- Bredemeyer AL, Sharma GG, Huang CY, Helmink BA, Walker LM, Khor KC, Nuskey B, Sullivan KE, Pandita TK, Bassing CH, et al. ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature. 2006;442:466–470. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

