Photogenerated lectin sensors produced by thiol-ene/yne photo-click chemistry in aqueous solution
- PMID: 22341757
- PMCID: PMC3405975
- DOI: 10.1016/j.bios.2012.01.001
Photogenerated lectin sensors produced by thiol-ene/yne photo-click chemistry in aqueous solution
Abstract
The photoinitiated radical reactions between thiols and alkenes/alkynes (thiol-ene and thiol-yne chemistry) have been applied to a functionalization methodology to produce carbohydrate-presenting surfaces for analyses of biomolecular interactions. Polymer-coated quartz surfaces were functionalized with alkenes or alkynes in a straightforward photochemical procedure utilizing perfluorophenylazide (PFPA) chemistry. The alkene/alkyne surfaces were subsequently allowed to react with carbohydrate thiols in water under UV-irradiation. The reaction can be carried out in a drop of water directly on the surface without photoinitiator, and any disulfide side products were easily washed away after the functionalization process. The resulting carbohydrate-presenting surfaces were evaluated in real-time studies of protein-carbohydrate interactions using a quartz crystal microbalance (QCM) flow-through system with recurring injections of selected lectins, with intermediate regeneration steps using low pH buffer. The resulting methodology proved fast, efficient and scalable to high-throughput analysis formats, and the produced surfaces showed significant protein binding with expected selectivities of the lectins used in the study.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures

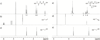

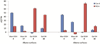
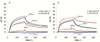

Similar articles
-
Monocatenary, branched, double-headed, and bolaform surface active carbohydrate esters via photochemical thiol-ene/-yne reactions.Carbohydr Res. 2013 Oct 18;380:29-36. doi: 10.1016/j.carres.2013.07.003. Epub 2013 Jul 15. Carbohydr Res. 2013. PMID: 23916852
-
Efficient functionalization of oxide-free silicon(111) surfaces: thiol-yne versus thiol-ene click chemistry.Langmuir. 2013 Apr 9;29(14):4535-42. doi: 10.1021/la400007y. Epub 2013 Mar 25. Langmuir. 2013. PMID: 23528051
-
Photo-click immobilization of carbohydrates on polymeric surfaces--a quick method to functionalize surfaces for biomolecular recognition studies.Bioconjug Chem. 2009 Dec;20(12):2364-70. doi: 10.1021/bc9003519. Bioconjug Chem. 2009. PMID: 19888719 Free PMC article.
-
Progress on Catalytic Systems Used in Click Polymerization.Macromol Rapid Commun. 2018 Jun;39(11):e1800098. doi: 10.1002/marc.201800098. Epub 2018 Apr 22. Macromol Rapid Commun. 2018. PMID: 29682849 Review.
-
Surface modification of cellulose via photo-induced click reaction.Carbohydr Polym. 2023 Feb 1;301(Pt B):120321. doi: 10.1016/j.carbpol.2022.120321. Epub 2022 Nov 11. Carbohydr Polym. 2023. PMID: 36446489 Review.
Cited by
-
Are glycan biosensors an alternative to glycan microarrays?Anal Methods. 2014 Sep 7;6(17):6610-6620. doi: 10.1039/C4AY00692E. Epub 2014 Jul 9. Anal Methods. 2014. PMID: 27231487 Free PMC article.
-
Synthesis of multifunctional cellulose nanocrystals for lectin recognition and bacterial imaging.Biomacromolecules. 2015 Apr 13;16(4):1426-32. doi: 10.1021/acs.biomac.5b00227. Epub 2015 Mar 12. Biomacromolecules. 2015. PMID: 25738860 Free PMC article.
-
Synthesis and Structure-Activity Relationship Study of Antimicrobial Auranofin against ESKAPE Pathogens.J Med Chem. 2019 Sep 12;62(17):7751-7768. doi: 10.1021/acs.jmedchem.9b00550. Epub 2019 Aug 21. J Med Chem. 2019. PMID: 31386365 Free PMC article.
-
Fabrication of Carbohydrate Chips Based on Polydopamine for Real-Time Determination of Carbohydrate⁻Lectin Interactions by QCM Biosensor.Polymers (Basel). 2018 Nov 16;10(11):1275. doi: 10.3390/polym10111275. Polymers (Basel). 2018. PMID: 30961200 Free PMC article.
-
Maltoheptaose-Presenting Nanoscale Glycoliposomes for the Delivery of Rifampicin to E. coli.ACS Appl Nano Mater. 2021 Jul 23;4(7):7343-7357. doi: 10.1021/acsanm.1c01320. Epub 2021 Jul 12. ACS Appl Nano Mater. 2021. PMID: 34746649 Free PMC article.
References
-
- Bader H. 23. The addition of thiolacetic acid to ethynylcarbinols and the conversion of the adducts into aldols and [small alpha][small beta]-unsaturated aldehydes. J. Chem. Soc. 1956:116–121.
-
- Bader H, Cross LC, Heilbron I, Jones ERH. 132. Researches on acetylenic compounds. Part XVIII. The addition of thiolacetic acid to acetylenic hydrocarbons. The conversion of monosubstituted acetylenes into aldehydes and 1 : 2-dithiols. J. Chem. Soc. 1949:619–623.
-
- Bhattacharyya L, Brewer CF. Isoelectric focusing studies of concanavalin A and the lentil lectin. J. Chromatogr. A. 1990;502:131–142. - PubMed
-
- Branderhorst HM, Ruijtenbeek R, Liskamp RMJ, Pieters RJ. Multivalent Carbohydrate Recognition on a Glycodendrimer-Functionalized Flow-Through Chip. ChemBioChem. 2008;9(11):1836–1844. - PubMed
-
- Chachadi VB, Inamdar SR, Yu L-G, Rhodes JM, Swamy BM. Exquisite binding specificity of Sclerotium rolfsii lectin toward TF-related O-linked mucin-type glycans. Glycoconjugate J. 2011;28(1):49–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

