A role for granulocyte-macrophage colony-stimulating factor in the regulation of CD8(+) T cell responses to rabies virus
- PMID: 22341782
- PMCID: PMC3294073
- DOI: 10.1016/j.virol.2012.01.025
A role for granulocyte-macrophage colony-stimulating factor in the regulation of CD8(+) T cell responses to rabies virus
Abstract
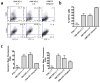
Inflammatory cytokines have a significant role in altering the innate and adaptive arms of immune responses. Here, we analyzed the effect of GM-CSF on a RABV-vaccine vector co-expressing HIV-1 Gag. To this end, we immunized mice with RABV expressing HIV-1 Gag and GM-CSF and analyzed the primary and recall CD8(+) T cell responses. We observed a statistically significant increase in antigen presenting cells (APCs) in the spleen and draining lymph nodes in response to GM-CSF. Despite the increase in APCs, the primary and memory anti HIV-1 CD8(+) T cell response was significantly lower. This was partly likely due to lower levels of proliferation in the spleen. Animals treated with GM-CSF neutralizing antibodies restored the CD8(+) T cell response. These data define a role of GM-CSF expression, in the regulation of the CD8(+) T cell immune responses against RABV and has implications in the use of GM-CSF as a molecular adjuvant in vaccine development.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures








Similar articles
-
Chimeric rabies virus-like particles containing membrane-anchored GM-CSF enhances the immune response against rabies virus.Viruses. 2015 Mar 11;7(3):1134-52. doi: 10.3390/v7031134. Viruses. 2015. PMID: 25768031 Free PMC article.
-
Rabies virus expressing dendritic cell-activating molecules enhances the innate and adaptive immune response to vaccination.J Virol. 2011 Feb;85(4):1634-44. doi: 10.1128/JVI.01552-10. Epub 2010 Nov 24. J Virol. 2011. PMID: 21106736 Free PMC article.
-
Comparative ability of various plasmid-based cytokines and chemokines to adjuvant the activity of HIV plasmid DNA vaccines.Vaccine. 2008 Sep 2;26(37):4819-29. doi: 10.1016/j.vaccine.2008.06.103. Epub 2008 Jul 25. Vaccine. 2008. PMID: 18657584 Free PMC article.
-
Intracerebral administration of recombinant rabies virus expressing GM-CSF prevents the development of rabies after infection with street virus.PLoS One. 2011;6(9):e25414. doi: 10.1371/journal.pone.0025414. Epub 2011 Sep 28. PLoS One. 2011. PMID: 21980450 Free PMC article.
-
Recombinant rabies viruses expressing GM-CSF or flagellin are effective vaccines for both intramuscular and oral immunizations.PLoS One. 2013 May 20;8(5):e63384. doi: 10.1371/journal.pone.0063384. Print 2013. PLoS One. 2013. PMID: 23700422 Free PMC article.
Cited by
-
Construction and in vitro evaluation of a recombinant live attenuated PRRSV expressing GM-CSF.Virol J. 2014 Nov 25;11:201. doi: 10.1186/s12985-014-0201-4. Virol J. 2014. PMID: 25420583 Free PMC article.
-
Transcriptional and posttranscriptional regulation of cytokine gene expression in HIV-1 antigen-specific CD8+ T cells that mediate virus inhibition.J Virol. 2014 Sep 1;88(17):9514-28. doi: 10.1128/JVI.00802-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899193 Free PMC article.
-
Viral vectored granulocyte-macrophage colony stimulating factor inhibits vaccine protection in an SIV challenge model: protection correlates with neutralizing antibody.Vaccine. 2012 Jun 13;30(28):4233-9. doi: 10.1016/j.vaccine.2012.04.046. Epub 2012 Apr 23. Vaccine. 2012. PMID: 22537983 Free PMC article.
-
Stable incorporation of GM-CSF into dissolvable microneedle patch improves skin vaccination against influenza.J Control Release. 2018 Apr 28;276:1-16. doi: 10.1016/j.jconrel.2018.02.033. Epub 2018 Feb 26. J Control Release. 2018. PMID: 29496540 Free PMC article.
-
Transgenic sickle cell disease mice have high mortality and dysregulated immune responses after vaccination.Pediatr Res. 2013 Aug;74(2):141-7. doi: 10.1038/pr.2013.85. Epub 2013 May 31. Pediatr Res. 2013. PMID: 23728384 Free PMC article.
References
-
- Bukreyev A, Belyakov IM, Berzofsky JA, Murphy BR, Collins PL. Granulocyte-macrophage colony-stimulating factor expressed by recombinant respiratory syncytial virus attenuates viral replication and increases the level of pulmonary antigen-presenting cells. J Virol. 2001;75:12128–12140. - PMC - PubMed
-
- Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977;252:1998–2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

