A randomized controlled pilot trial of oral N-acetylcysteine in children with autism
- PMID: 22342106
- PMCID: PMC4914359
- DOI: 10.1016/j.biopsych.2012.01.014
A randomized controlled pilot trial of oral N-acetylcysteine in children with autism
Abstract
Background: An imbalance in the excitatory/inhibitory systems with abnormalities in the glutamatergic pathways has been implicated in the pathophysiology of autism. Furthermore, chronic redox imbalance was also recently linked to this disorder. The goal of this pilot study was to assess the feasibility of using oral N-acetylcysteine (NAC), a glutamatergic modulator and an antioxidant, in the treatment of behavioral disturbance in children with autism.
Methods: This was a 12-week, double-blind, randomized, placebo-controlled study of NAC in children with autistic disorder. Subjects randomized to NAC were initiated at 900 mg daily for 4 weeks, then 900 mg twice daily for 4 weeks and 900 mg three times daily for 4 weeks. The primary behavioral measure (Aberrant Behavior Checklist [ABC] irritability subscale) and safety measures were performed at baseline and 4, 8, and 12 weeks. Secondary measures included the ABC stereotypy subscale, Repetitive Behavior Scale-Revised, and Social Responsiveness Scale.
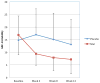
Results: Thirty-three subjects (31 male subjects, 2 female subjects; aged 3.2-10.7 years) were randomized in the study. Follow-up data was available on 14 subjects in the NAC group and 15 in the placebo group. Oral NAC was well tolerated with limited side effects. Compared with placebo, NAC resulted in significant improvements on ABC irritability subscale (F = 6.80; p < .001; d = .96).
Conclusions: Data from this pilot investigation support the potential usefulness of NAC for treating irritability in children with autistic disorder. Large randomized controlled investigations are warranted.
Trial registration: ClinicalTrials.gov NCT00627705.
Copyright © 2012 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Translating the Rosetta Stone of N-acetylcysteine.Biol Psychiatry. 2012 Jun 1;71(11):935-6. doi: 10.1016/j.biopsych.2012.04.001. Biol Psychiatry. 2012. PMID: 22579303 No abstract available.
References
-
- Belmonte MK, Cook EH, Jr, Anderson GM, Rubenstein JL, Greenough WT, Beckel-Mitchener A, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry. 2004;9:646–663. - PubMed
-
- Kern JK, Jones AM. Evidence of toxicity, oxidative stress, and neuronal insult in autism. J Toxicol Environ Health B Crit Rev. 2006;9:485–499. - PubMed
-
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

