Patient-calibrated agent-based modelling of ductal carcinoma in situ (DCIS): from microscopic measurements to macroscopic predictions of clinical progression
- PMID: 22342935
- PMCID: PMC3322268
- DOI: 10.1016/j.jtbi.2012.02.002
Patient-calibrated agent-based modelling of ductal carcinoma in situ (DCIS): from microscopic measurements to macroscopic predictions of clinical progression
Abstract
Ductal carcinoma in situ (DCIS)--a significant precursor to invasive breast cancer--is typically diagnosed as microcalcifications in mammograms. However, the effective use of mammograms and other patient data to plan treatment has been restricted by our limited understanding of DCIS growth and calcification. We develop a mechanistic, agent-based cell model and apply it to DCIS. Cell motion is determined by a balance of biomechanical forces. We use potential functions to model interactions with the basement membrane and amongst cells of unequal size and phenotype. Each cell's phenotype is determined by genomic/proteomic- and microenvironment-dependent stochastic processes. Detailed "sub-models" describe cell volume changes during proliferation and necrosis; we are the first to account for cell calcification. We introduce the first patient-specific calibration method to fully constrain the model based upon clinically-accessible histopathology data. After simulating 45 days of solid-type DCIS with comedonecrosis, the model predicts: necrotic cell lysis acts as a biomechanical stress relief and is responsible for the linear DCIS growth observed in mammography; the rate of DCIS advance varies with the duct radius; the tumour grows 7-10mm per year--consistent with mammographic data; and the mammographic and (post-operative) pathologic sizes are linearly correlated--in quantitative agreement with the clinical literature. Patient histopathology matches the predicted DCIS microstructure: an outer proliferative rim surrounds a stratified necrotic core with nuclear debris on its outer edge and calcification in the centre. This work illustrates that computational modelling can provide new insight on the biophysical underpinnings of cancer. It may 1-day be possible to augment a patient's mammography and other imaging with rigorously-calibrated models that help select optimal surgical margins based upon the patient's histopathologic data.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures









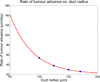



Comment in
-
Improved patient-specific calibration for agent-based cancer modeling.J Theor Biol. 2013 Jan 21;317:422-4. doi: 10.1016/j.jtbi.2012.10.017. Epub 2012 Oct 22. J Theor Biol. 2013. PMID: 23084996 Free PMC article. No abstract available.
References
-
- Abbott RG, Forrest S, Pienta KJ. Simulating the hallmarks of cancer. Artif. Life. 2006;12(4):617–634. - PubMed
-
- American Cancer Society. Atlanta: American Cancer Society, Inc.; 2007. American cancer society breast cancer facts and figures 2007–2008.
-
- Astanin S, Preziosi L. Mathematical modelling of the warburg effect in tumour cords. J. Theor. Biol. 2009;254(4):578–590. - PubMed
-
- Ayre KJ, Hulbert AJ. Dietary fatty acid profile influences the composition of skeletal muscle phospholipids in rats. J. Nutr. 1996;126(3):653–662. - PubMed
-
- Bankhead A, III, Magnuson NS, Heckendorn RB. Cellular automaton simulation examining progenitor heirarchy structure effects on mammary ductal carcinoma in situ. J. Theor. Biol. 2007;246(3):491–498. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

