DnaJA1 antagonizes constitutive Hsp70-mediated stabilization of tau
- PMID: 22343013
- PMCID: PMC3371317
- DOI: 10.1016/j.jmb.2012.02.003
DnaJA1 antagonizes constitutive Hsp70-mediated stabilization of tau
Abstract
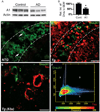
Tau aggregation and amyloidogenesis are common hallmarks for neurodegenerative disorders called tauopathies. The molecular chaperone network constitutes the cellular defense against insults such as tau aggregation. However, chaperone effects on tau are dichotomous. Loss of tau's microtubule-binding activity facilitates an inappropriate chaperone interaction that promotes an amyloidogenic tau conformation. Conversely, other chaperones are capable of promoting tau clearance. Here, we demonstrate that a critical contributor to tau triage is the DnaJ-binding domain of Hsp70 proteins. In particular, over-expression of the constitutive DnaJ, DnaJA1, mediated tau clearance, while knockdown facilitated tau accumulation. This clearance was not specific to distinct pathogenic tau species. The activity of DnaJA1 was attenuated by concomitant increases in Hsp70. Tau reductions facilitated by DnaJA1 were dependent on the integrity of lysines known to be poly-ubiquitinated in human Alzheimer's brain. In vivo, DnaJA1 and tau levels were inversely correlated. The effects of DnaJA1 were partially specific: DnaJA1 reduced the levels of a polyQ protein but had no significant effect on α-synuclein levels. These data suggest that DnaJA1 triages all tau species for ubiquitin-dependent clearance mechanisms. Moreover, the levels of DnaJA1 and Hsp70 seem to play against each other with regard to tau: as DnaJA1 levels increase, tau levels are reduced, but this can be prevented if Hsp70 levels are simultaneously induced. Thus, the DnaJ repertoire possibly represents a powerful set of genetic modifiers for tau pathogenesis. Further investigations could provide new insights about triage decisions that facilitate or prevent amyloidogenesis of tau and other proteins associated with neurodegenerative disease.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures




References
-
- Hatahet F, Ruddock LW. Modulating proteostasis: peptidomimetic inhibitors and activators of protein folding. Curr Pharm Des. 2009;15:2488–2507. - PubMed
-
- Hardy J, Pittman A, Myers A, Fung HC, de Silva R, Duckworth J. Tangle diseases and the tau haplotypes. Alzheimer Dis Assoc Disord. 2006;20:60–62. - PubMed
-
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. - PubMed
-
- Walter S, Buchner J. Molecular chaperones--cellular machines for protein folding. Angew Chem Int Ed Engl. 2002;41:1098–1113. - PubMed
-
- Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

