Early basal insulin therapy decreases new-onset diabetes after renal transplantation
- PMID: 22343119
- PMCID: PMC3312499
- DOI: 10.1681/ASN.2011080835
Early basal insulin therapy decreases new-onset diabetes after renal transplantation
Abstract
No effective interventions to reduce risk for new-onset diabetes after transplantation (NODAT), a condition associated with postoperative hyperglycemia and reduced patient and graft survival, have been established. In this 1-year, proof-of-concept clinical trial, we randomly assigned 50 renal transplant recipients to immediate-postoperative isophane insulin for evening blood glucose ≥140 mg/dl (treatment group) or short-acting insulin and/or oral antidiabetic agents for blood glucose ≥180-250 mg/dl (standard-of-care control group). We included only patients without a history of diabetes who received tacrolimus. By the third postoperative evening, all patients in the treatment group had blood glucose ≥140 mg/dl and were subsequently treated with basal insulin; during the first 3 weeks after transplantation, the mean ± SD daily insulin dosage was 17±11 IU/d. Among controls, 23 (92%) of 25 had blood glucose ≥200 mg/dl and 18 (72%) of 25 received standard-of-care antihyperglycemic treatment. Asymptomatic hypoglycemia occurred five times in the treatment group and once in the control group. Throughout follow-up, the treatment group had 73% lower odds of NODAT (odds ratio, 0.27) than the control group, and hemoglobin A1c was on average 0.38% lower in the treatment group than the control group. Twelve months after transplantation, all patients in the treatment group were insulin-independent, whereas 7 (28%) of 25 controls required antidiabetic agents. The groups did not differ for insulin sensitivity, but the treatment group showed better β-cell function throughout the 1-year follow-up. In conclusion, this study suggests regimens that include basal insulin significantly reduce the odds for NODAT after renal transplantation, presumably via insulin-mediated protection of β cells.
Figures





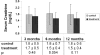
Comment in
-
Transplantation: Basal insulin therapy may reduce the risk of new-onset diabetes after renal transplantation.Nat Rev Nephrol. 2012 Mar 13;8(4):191. doi: 10.1038/nrneph.2012.20. Nat Rev Nephrol. 2012. PMID: 22410491 No abstract available.
References
-
- Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 - PubMed
-
- Krentz AJ, Wheeler DC: New-onset diabetes after transplantation: A threat to graft and patient survival. Lancet 365: 640–642, 2005 - PubMed
-
- Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, Woodworth TG, Brennan DC: Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant 3: 590–598, 2003 - PubMed
-
- Hjelmesaeth J, Hartmann A, Leivestad T, Holdaas H, Sagedal S, Olstad M, Jenssen T: The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int 69: 588–595, 2006 - PubMed
-
- Cosio FG, Kudva Y, van der Velde M, Larson TS, Textor SC, Griffin MD, Stegall MD: New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int 67: 2415–2421, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

