Nxnl2 splicing results in dual functions in neuronal cell survival and maintenance of cell integrity
- PMID: 22343139
- PMCID: PMC3664437
- DOI: 10.1093/hmg/dds050
Nxnl2 splicing results in dual functions in neuronal cell survival and maintenance of cell integrity
Abstract
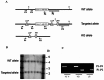
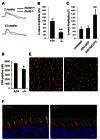
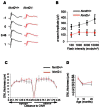
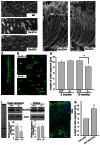
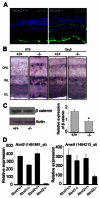
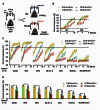
The rod-derived cone viability factors, RdCVF and RdCVF2, have potential therapeutical interests for the treatment of inherited photoreceptor degenerations. In the mouse lacking Nxnl2, the gene encoding RdCVF2, the progressive decline of the visual performance of the cones in parallel with their degeneration, arises due to the loss of trophic support from RdCVF2. In contrary, the progressive loss of rod visual function of the Nxnl2-/- mouse results from a decrease in outer segment length, mediated by a cell autonomous mechanism involving the putative thioredoxin protein RdCVF2L, the second spliced product of the Nxnl2 gene. This novel signaling mechanism extends to olfaction as shown by the progressive impairment of olfaction in aged Nxnl2-/- mice and the protection of olfactory neurons by RdCVF2. This study shows that Nxnl2 is a bi-functional gene involved in the maintenance of both the function and the viability of sensory neurons.
Conflict of interest statement
CJ, JAS and TL have a patent on
Figures


References
-
- Léveillard T, Mohand-Said S, Lorentz O, Hicks D, Fintz AC, Clerin E, Simonutti M, Forster V, Cavusoglu N, Chalmel F, et al. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36:755–759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

