Long-term effects of alemtuzumab on regulatory and memory T-cell subsets in kidney transplantation
- PMID: 22343334
- PMCID: PMC3323763
- DOI: 10.1097/TP.0b013e318247a717
Long-term effects of alemtuzumab on regulatory and memory T-cell subsets in kidney transplantation
Abstract
Background: Induction with lymphocyte-depleting antibodies is routinely used to prevent rejection but often skews T cells toward memory. It is not fully understood which memory and regulatory T-cell subsets are most affected and how they relate to clinical outcomes.
Methods: We analyzed T cells from 57 living-donor renal transplant recipients (12 reactive and 45 quiescent) 2.8±1.4 years after alemtuzumab induction. Thirty-four healthy subjects and nine patients with acute cellular rejection (ACR) were also studied.
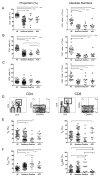
Results: We found that alemtuzumab caused protracted CD4 more than CD8 T-lymphocyte deficiency, increased proportion of CD4 memory T cells, and decreased proportion of CD4 regulatory T cells. Reactive patients exhibited higher proportions of CD4 effector memory T cells (TEM) and CD8 terminally differentiated TEM (TEMRA), with greater CD4 TEM and CD8 TEMRA to regulatory T cell ratios, than quiescent patients or healthy controls. Patients with ongoing ACR had profound reduction in circulating CD8 TEMRA. Mixed lymphocyte assays showed significantly lower T-cell proliferation to donor than third-party antigens in the quiescent group, while reactive and ACR patients exhibited increased effector molecules in CD8 T cells.
Conclusions: Our findings provide evidence that T-cell skewing toward TEM may be associated with antigraft reactivity long after lymphodepletion. Further testing of TEM and TEMRA subsets as rejection predictors is warranted.
Conflict of interest statement
All authors declare no conflict of interest.
Figures



References
-
- Cai J, Terasaki PI. Induction Immunosuppression Improves Long-Term Graft and Patient Outcome in Organ Transplantation: An Analysis of United Network for Organ Sharing Registry Data. Transplantation. 2010;90:1511. - PubMed
-
- Meier-Kriesche H-U, Li S, Gruessner RWG, et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6 (5 Pt 2):1111. - PubMed
-
- Kirk AD. Induction immunosuppression. Transplantation. 2006;82 (5):593. - PubMed
-
- Margreiter R, Klempnauer J, Neuhaus P, Muehlbacher F, Boesmueller C, Calne RY. Alemtuzumab (Campath-1H) and tacrolimus monotherapy after renal transplantation: results of a prospective randomized trial. Am J Transplant. 2008;8 (7):1480. - PubMed
-
- Kaufman DB, Leventhal JR, Gallon LG, Parker MA. Alemtuzumab induction and prednisone-free maintenance immunotherapy in simultaneous pancreas-kidney transplantation comparison with rabbit antithymocyte globulin induction - long-term results. Am J Transplant. 2006;6 (2):331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

