OLIG2 over-expression impairs proliferation of human Down syndrome neural progenitors
- PMID: 22343408
- PMCID: PMC3335315
- DOI: 10.1093/hmg/dds052
OLIG2 over-expression impairs proliferation of human Down syndrome neural progenitors
Abstract
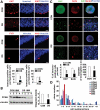
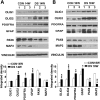
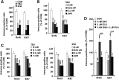
Mental retardation and early Alzheimer's disease (AD) have generally been attributed to progressive neuronal loss in the developing and mature Down syndrome (DS) brain. However, reduced neuronal production during development could also contribute to the smaller brain size and simplified gyral patterning seen in this disorder. Here, we show impairments in proliferation within the ventricular zone (VZ) of early DS fetal cortex and in cultured early passage DS human neural progenitors (HNPs). We find that the reduced proliferative rates correspond temporally with increased expression of the chromosome 21 (HSA21) associated, oligodendrocyte transcription factor OLIG2 at 14-18 weeks gestational age (GA) (period of neurogenesis). Moreover, the DS HNPs adopt more oligodendrocyte-specific features including increased oligodendrocyte marker expression, as well as a reduction in KCNA3 potassium channel expression and function. We further show that OLIG2 inhibition or over-expression regulates potassium channel expression levels and that activation or inhibition of these channels influences the rate of progenitor proliferation. Finally, neural progenitors from Olig2 over-expressing transgenic mice exhibit these same impairments in proliferation and potassium channel expression. These findings suggest that OLIG2 over-expression inhibits neural progenitor proliferation through changes in potassium channel activity, thereby contributing to the reduced neuronal numbers and brain size in DS.
Figures


References
-
- Golden J.A., Hyman B.T. Development of the superior temporal neocortex is anomalous in trisomy 21. J. Neuropathol. Exp. Neurol. 1994;53:513–520. - PubMed
-
- Esposito G., Imitola J., Lu J., De Filippis D., Scuderi C., Ganesh V.S., Folkerth R., Hecht J., Shin S., Iuvone T., et al. Genomic and functional profiling of human Down syndrome neural progenitors implicates S100B and aquaporin 4 in cell injury. Hum. Mol. Genet. 2008;17:440–457. - PubMed
-
- Contestabile A., Fila T., Ceccarelli C., Bonasoni P., Bonapace L., Santini D., Bartesaghi R., Ciani E. Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with Down syndrome and in Ts65Dn mice. Hippocampus. 2007;17:665–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

