Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation
- PMID: 22343625
- PMCID: PMC3320953
- DOI: 10.1074/jbc.M111.316463
Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation
Abstract
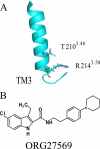
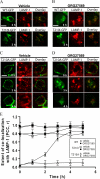
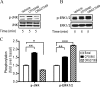
The cannabinoid receptor 1 (CB1), a member of the class A G protein-coupled receptor family, is expressed in brain tissue where agonist stimulation primarily activates the pertussis toxin-sensitive inhibitory G protein (G(i)). Ligands such as CP55940 ((1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3- hydroxypropyl)cyclohexan-1-ol) and Δ(9)-tetrahydrocannabinol are orthosteric agonists for the receptor, bind the conventional binding pocket, and trigger G(i)-mediated effects including inhibition of adenylate cyclase. ORG27569 (5-chloro-3-ethyl-1H-indole-2-carboxylic acid [2-(4-piperidin-1-yl-phenyl)ethyl]amide) has been identified as an allosteric modulator that displays positive cooperativity for CP55940 binding to CB1 yet acts as an antagonist of G protein coupling. To examine this apparent conundrum, we used the wild-type CB1 and two mutants, T210A and T210I (D'Antona, A. M., Ahn, K. H., and Kendall, D. A. (2006) Biochemistry 45, 5606-5617), which collectively cover a spectrum of receptor states from inactive to partially active to more fully constitutively active. Using these receptors, we demonstrated that ORG27569 induces a CB1 receptor state that is characterized by enhanced agonist affinity and decreased inverse agonist affinity consistent with an active conformation. Also consistent with this conformation, the impact of ORG27569 binding was most dramatic on the inactive T210A receptor and less pronounced on the already active T210I receptor. Although ORG27569 antagonized CP55940-induced guanosine 5'-3-O-(thio)triphosphate binding, which is indicative of G protein coupling inhibition in a concentration-dependent manner, the ORG27569-induced conformational change of the CB1 receptor led to cellular internalization and downstream activation of ERK signaling, providing the first case of allosteric ligand-biased signaling via CB1. ORG27569-induced ERK phosphorylation persisted even after pertussis toxin treatment to abrogate G(i) and occurs in HEK293 and neuronal cells.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

