Refinement of structural leads for centrally acting oxime reactivators of phosphylated cholinesterases
- PMID: 22343626
- PMCID: PMC3320928
- DOI: 10.1074/jbc.M111.333732
Refinement of structural leads for centrally acting oxime reactivators of phosphylated cholinesterases
Erratum in
- J Biol Chem. 2012 Jun 1;287(23):19337
Abstract
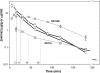
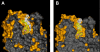
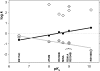
We present a systematic structural optimization of uncharged but ionizable N-substituted 2-hydroxyiminoacetamido alkylamine reactivators of phosphylated human acetylcholinesterase (hAChE) intended to catalyze the hydrolysis of organophosphate (OP)-inhibited hAChE in the CNS. Starting with the initial lead oxime RS41A identified in our earlier study and extending to the azepine analog RS194B, reactivation rates for OP-hAChE conjugates formed by sarin, cyclosarin, VX, paraoxon, and tabun are enhanced severalfold in vitro. To analyze the mechanism of intrinsic reactivation of the OP-AChE conjugate and penetration of the blood-brain barrier, the pH dependence of the oxime and amine ionizing groups of the compounds and their nucleophilic potential were examined by UV-visible spectroscopy, (1)H NMR, and oximolysis rates for acetylthiocholine and phosphoester hydrolysis. Oximolysis rates were compared in solution and on AChE conjugates and analyzed in terms of the ionization states for reactivation of the OP-conjugated AChE. In addition, toxicity and pharmacokinetic studies in mice show significantly improved CNS penetration and retention for RS194B when compared with RS41A. The enhanced intrinsic reactivity against the OP-AChE target combined with favorable pharmacokinetic properties resulted in great improvement of antidotal properties of RS194B compared with RS41A and the standard peripherally active oxime, 2-pyridinealdoxime methiodide. Improvement was particularly noticeable when pretreatment of mice with RS194B before OP exposure was combined with RS194B reactivation therapy after the OP insult.
Figures

References
-
- Mercey G., Verdelet T., Saint-André G., Gillon E., Wagner A., Baati R., Jean L., Nachon F., Renard P. Y. (2011) First efficient uncharged reactivators for the dephosphylation of poisoned human acetylcholinesterase. Chem. Commun. 475, 5295–5297 - PubMed
-
- de Koning M. C., van Grol M., Noort D. (2011) Peripheral site ligand conjugation to a non-quaternary oxime enhances reactivation of nerve agent-inhibited human acetylcholinesterase. Toxicol. Lett. 206, 54–59 - PubMed
-
- Kalisiak J., Ralph E. C., Zhang J., Cashman J. R. (2011) Amidine-oximes. Reactivators for organophosphate exposure. J. Med Chem. 54, 3319–3330 - PubMed
-
- Demar J. C., Clarkson E. D., Ratcliffe R. H., Campbell A. J., Thangavelu S. G., Herdman C. A., Leader H., Schulz S. M., Marek E., Medynets M. A., Ku T. C., Evans S. A., Khan F. A., Owens R. R., Nambiar M. P., Gordon R. K. (2010) Pro-2-PAM therapy for central and peripheral cholinesterases. Chem. Biol. Interact. 187, 191–198 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

