Anatomical and functional assessment of brown adipose tissue by magnetic resonance imaging
- PMID: 22343821
- PMCID: PMC4383098
- DOI: 10.1038/oby.2012.22
Anatomical and functional assessment of brown adipose tissue by magnetic resonance imaging
Abstract
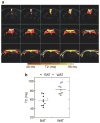
Brown adipose tissue (BAT) is the primary tissue responsible for nonshivering thermogenesis in mammals. The amount of BAT and its level of activation help regulate the utilization of excessive calories for thermogenesis as opposed to storage in white adipose tissue (WAT) which would lead to weight gain. Over the past several years, BAT activity in vivo has been primarily assessed by positron emission tomography-computed tomography (PET-CT) scan using 2-[18F]-fluoro-2-deoxy-D-glucose (18F-FDG) to measure glucose utilization associated with BAT mitochondrial respiration. In this study, we demonstrate the feasibility of mapping and estimating BAT volume and metabolic function in vivo in rats at a 9.4T magnetic resonance imaging (MRI) scanner using sequences available from clinical MR scanners. Based on the morphological characteristics of BAT, we measured the volume distribution of BAT with MRI sequences that have strong fat-water contrast. We also investigated BAT volume by utilizing spin-echo MRI sequences. The in vivo MRI-estimated BAT volumes were correlated with direct measurement of BAT mass from dissected samples. Using MRI, we also were able to map hemodynamic responses to changes in BAT metabolism induced pharmacologically by β3-adrenergic receptor agonist, CL-316,243 and compare this to BAT activity in response to CL-316,243 assessed by PET 18F-FDG. In conclusion, we demonstrate the feasibility of measuring BAT volume and function in vivo using routine MRI sequences. The MRI measurement of BAT volume is consistent with quantitative measurement of the tissue ex vivo.
Conflict of interest statement
The authors declared no conflict of interest.
Figures



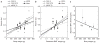


References
-
- Kozak LP, Koza RA, Anunciado-Koza R. Brown fat thermogenesis and body weight regulation in mice: relevance to humans. Int J Obes (Lond) 2010;34(Suppl 1):S23–S27. - PubMed
-
- Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. - PubMed
-
- Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. - PubMed
-
- Nedergaard J, Golozoubova V, Matthias A, et al. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504:82–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

