Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation
- PMID: 22343896
- PMCID: PMC3656605
- DOI: 10.1038/nature10898
Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation
Abstract
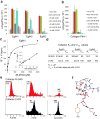
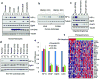
The identification of succinate dehydrogenase (SDH), fumarate hydratase (FH) and isocitrate dehydrogenase (IDH) mutations in human cancers has rekindled the idea that altered cellular metabolism can transform cells. Inactivating SDH and FH mutations cause the accumulation of succinate and fumarate, respectively, which can inhibit 2-oxoglutarate (2-OG)-dependent enzymes, including the EGLN prolyl 4-hydroxylases that mark the hypoxia inducible factor (HIF) transcription factor for polyubiquitylation and proteasomal degradation. Inappropriate HIF activation is suspected of contributing to the pathogenesis of SDH-defective and FH-defective tumours but can suppress tumour growth in some other contexts. IDH1 and IDH2, which catalyse the interconversion of isocitrate and 2-OG, are frequently mutated in human brain tumours and leukaemias. The resulting mutants have the neomorphic ability to convert 2-OG to the (R)-enantiomer of 2-hydroxyglutarate ((R)-2HG). Here we show that (R)-2HG, but not (S)-2HG, stimulates EGLN activity, leading to diminished HIF levels, which enhances the proliferation and soft agar growth of human astrocytes. These findings define an enantiomer-specific mechanism by which the (R)-2HG that accumulates in IDH mutant brain tumours promotes transformation and provide a justification for exploring EGLN inhibition as a potential treatment strategy.
Conflict of interest statement
Figures


Comment in
-
Metabolism: unmasking an oncometabolite.Nat Rev Cancer. 2012 Mar 1;12(4):229. doi: 10.1038/nrc3248. Nat Rev Cancer. 2012. PMID: 22378191 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

