Human mitotic chromosomes consist predominantly of irregularly folded nucleosome fibres without a 30-nm chromatin structure
- PMID: 22343941
- PMCID: PMC3321210
- DOI: 10.1038/emboj.2012.35
Human mitotic chromosomes consist predominantly of irregularly folded nucleosome fibres without a 30-nm chromatin structure
Abstract
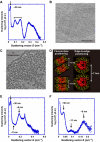
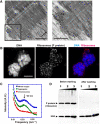
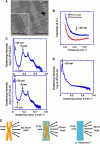
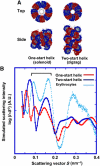
How a long strand of genomic DNA is compacted into a mitotic chromosome remains one of the basic questions in biology. The nucleosome fibre, in which DNA is wrapped around core histones, has long been assumed to be folded into a 30-nm chromatin fibre and further hierarchical regular structures to form mitotic chromosomes, although the actual existence of these regular structures is controversial. Here, we show that human mitotic HeLa chromosomes are mainly composed of irregularly folded nucleosome fibres rather than 30-nm chromatin fibres. Our comprehensive and quantitative study using cryo-electron microscopy and synchrotron X-ray scattering resolved the long-standing contradictions regarding the existence of 30-nm chromatin structures and detected no regular structure >11 nm. Our finding suggests that the mitotic chromosome consists of irregularly arranged nucleosome fibres, with a fractal nature, which permits a more dynamic and flexible genome organization than would be allowed by static regular structures.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Comment in
-
Human mitotic chromosome structure: what happened to the 30-nm fibre?EMBO J. 2012 Apr 4;31(7):1621-3. doi: 10.1038/emboj.2012.66. Epub 2012 Mar 13. EMBO J. 2012. PMID: 22415369 Free PMC article.
-
A silent revolution in chromosome biology.Nat Rev Mol Cell Biol. 2014 Jul;15(7):431. doi: 10.1038/nrm3817. Epub 2014 May 29. Nat Rev Mol Cell Biol. 2014. PMID: 24871800 No abstract available.
References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2007) Molecular Biology of the Cell, Fifth Edition. New York: Garland Science
-
- Bordas J, Perez-Grau L, Koch MH, Vega MC, Nave C (1986) The superstructure of chromatin and its condensation mechanism. II. Theoretical analysis of the X-ray scattering patterns and model calculations. Eur Biophys J 13: 175–185 - PubMed
-
- Conway JF, Steven AC (1999) Methods for reconstructing density maps of ‘single’ particles from cryoelectron micrographs to subnanometer resolution. J Struct Biol 128: 106–118 - PubMed
-
- Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ (2002) Solvent mediated interactions in the structure of the nucleosome core particle at 1.9Å resolution. J Mol Biol 319: 1097–1113 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

