Supervillin couples myosin-dependent contractility to podosomes and enables their turnover
- PMID: 22344260
- PMCID: PMC3367944
- DOI: 10.1242/jcs.100032
Supervillin couples myosin-dependent contractility to podosomes and enables their turnover
Abstract
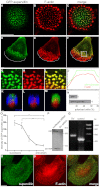
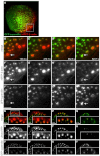
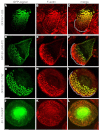
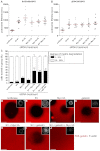
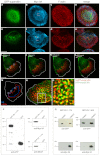
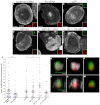
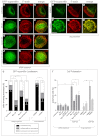
Podosomes are actin-rich adhesion and invasion structures. Especially in macrophages, podosomes exist in two subpopulations, large precursors at the cell periphery and smaller podosomes (successors) in the cell interior. To date, the mechanisms that differentially regulate these subpopulations are largely unknown. Here, we show that the membrane-associated protein supervillin localizes preferentially to successor podosomes and becomes enriched at precursors immediately before their dissolution. Consistently, podosome numbers are inversely correlated with supervillin protein levels. Using deletion constructs, we find that the myosin II regulatory N-terminus of supervillin [SV(1-174)] is crucial for these effects. Phosphorylated myosin light chain (pMLC) localizes at supervillin-positive podosomes, and time-lapse analyses show that enrichment of GFP-supervillin at podosomes coincides with their coupling to contractile myosin-IIA-positive cables. We also show that supervillin binds only to activated myosin IIA, and a dysregulated N-terminal construct [SV(1-830)] enhances pMLC levels at podosomes. Thus, preferential recruitment of supervillin to podosome subpopulations might both require and induce actomyosin contractility. Using siRNA and pharmacological inhibition, we demonstrate that supervillin and myosin IIA cooperate to regulate podosome lifetime, podosomal matrix degradation and cell polarization. In sum, we show here that podosome subpopulations differ in their molecular composition and identify supervillin, in cooperation with myosin IIA, as a crucial factor in the regulation of podosome turnover and function.
Figures


References
-
- Abramoff M. D., Magalhaes P. J., Ram S. J. (2004). Image processing with ImageJ. Biophotonics International 11, 36-42
-
- Blanchoin L., Pollard T. D., Hitchcock-DeGregori S. E. (2001). Inhibition of the Arp2/3 complex-nucleated actin polymerization and branch formation by tropomyosin. Curr. Biol. 11, 1300-1304 - PubMed
-
- Bowden E. T., Onikoyi E., Slack R., Myoui A., Yoneda T., Yamada K. M., Mueller S. C. (2006). Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp. Cell Res. 312, 1240-1253 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

