Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium
- PMID: 22344263
- PMCID: PMC3403234
- DOI: 10.1242/jcs.096875
Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium
Abstract
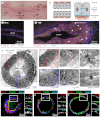
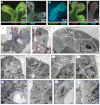
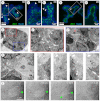
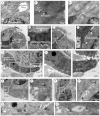
Normal mammary morphogenesis involves transitions between simple and multilayered epithelial organizations. We used electron microscopy and molecular markers to determine whether intercellular junctions and apico-basal polarity were maintained in the multilayered epithelium. We found that multilayered elongating ducts had polarized apical and basal tissue surfaces both in three-dimensional culture and in vivo. However, individual cells were only polarized on surfaces in contact with the lumen or extracellular matrix. The basolateral marker scribble and the apical marker atypical protein kinase C zeta localized to all interior cell membranes, whereas PAR3 displayed a cytoplasmic localization, suggesting that the apico-basal polarity was incomplete. Despite membrane localization of E-cadherin and β-catenin, we did not observe a defined zonula adherens connecting interior cells. Instead, interior cells were connected through desmosomes and exhibited complex interdigitating membrane protrusions. Single-cell labeling revealed that individual cells were both protrusive and migratory within the epithelial multilayer. Inhibition of Rho kinase (ROCK) further reduced intercellular adhesion on apical and lateral surfaces but did not disrupt basal tissue organization. Following morphogenesis, segregated membrane domains were re-established and junctional complexes re-formed. We observed similar epithelial organization during mammary morphogenesis in organotypic culture and in vivo. We conclude that mammary epithelial morphogenesis involves a reversible, spatially limited, reduction in polarity and intercellular junctions and active individualistic cell migration. Our data suggest that reductions in polarity and adhesion during breast cancer progression might reflect partial recapitulation of a normal developmental program.
Figures


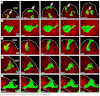
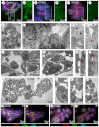


References
-
- Berx G., Cleton-Jansen A. M., Strumane K., de Leeuw W. J., Nollet F., van Roy F., Cornelisse C. (1996). E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene 13, 1919-1925 - PubMed
-
- Bilder D., Li M., Perrimon N. (2000). Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113-116 - PubMed
-
- Bogenrieder T., Herlyn M. (2003). Axis of evil: molecular mechanisms of cancer metastasis. Oncogene 22, 6524-6536 - PubMed
-
- Chepko G., Smith G. H. (1997). Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell 29, 239-253 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

