MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways
- PMID: 22344686
- PMCID: PMC3672221
- DOI: 10.1126/scitranslmed.3003205
MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways
Abstract
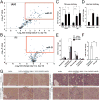
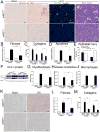
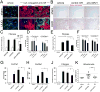
Scarring of the kidney is a major public health concern, directly promoting loss of kidney function. To understand the role of microRNA (miRNA) in the progression of kidney scarring in response to injury, we investigated changes in miRNA expression in two kidney fibrosis models and identified 24 commonly up-regulated miRNAs. Among them, miR-21 was highly elevated in both animal models and in human transplanted kidneys with nephropathy. Deletion of miR-21 in mice resulted in no overt abnormality. However, miR-21(-/-) mice suffered far less interstitial fibrosis in response to kidney injury, a phenotype duplicated in wild-type mice treated with anti-miR-21 oligonucleotides. Global derepression of miR-21 target mRNAs was readily detectable in miR-21(-/-) kidneys after injury. Analysis of gene expression profiles up-regulated in the absence of miR-21 identified groups of genes involved in metabolic pathways, including the lipid metabolism pathway regulated by peroxisome proliferator-activated receptor-α (Pparα), a direct miR-21 target. Overexpression of Pparα prevented ureteral obstruction-induced injury and fibrosis. Pparα deficiency abrogated the antifibrotic effect of anti-miR-21 oligonucleotides. miR-21 also regulated the redox metabolic pathway. The mitochondrial inhibitor of reactive oxygen species generation Mpv17l was repressed by miR-21, correlating closely with enhanced oxidative kidney damage. These studies demonstrate that miR-21 contributes to fibrogenesis and epithelial injury in the kidney in two mouse models and is a candidate target for antifibrotic therapies.
Conflict of interest statement
Competing interests: All authors from Regulus Therapeutics have stock options in the company. JSD is on the Scientific Advisory Board at Regulus Therapeutics and the Duffield Lab has a Sponsored Research Agreement with Regulus Therapeutics. JSD is on the Scientific Advisory Board at, and has stock options in, Promedior Inc.
Figures



References
-
- Lupher ML, Jr, Gallatin WM. Regulation of fibrosis by the immune system. Adv Immunol. 2006;89:245–288. - PubMed
-
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. - PubMed
-
- Thum T, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

