Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation
- PMID: 22345431
- PMCID: PMC3362236
- DOI: 10.1152/japplphysiol.00027.2012
Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation
Abstract
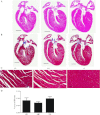
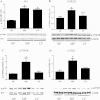
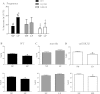
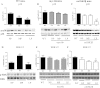
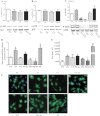
Although the signaling pathways underlying exercise-induced cardiac adaptation have been extensively studied, little is known about the molecular mechanisms that result in the response of the heart to pregnancy. The objective of this study was to define the morphological, functional, and gene expression patterns that define the hearts of pregnant mice, and to identify the signaling pathways that mediate this response. Mice were divided into three groups: nonpregnant diestrus control, midpregnancy, and late pregnancy. Both time points of pregnancy were associated with significant cardiac hypertrophy. The prosurvival signaling cascades of Akt and ERK1/2 were activated in the hearts of pregnant mice, while the stress kinase, p38, was decreased. Given the activation of Akt in pregnancy and its known role in cardiac hypertrophy, the hypertrophic response to pregnancy was tested in mice expressing a cardiac-specific activated (myristoylated) form of Akt (myrAkt) or a cardiac-specific constitutively active (antipathologic hypertrophic) form of its downstream target, glycogen synthase kinase 3β (caGSK3β). The pregnancy-induced hypertrophic responses of hearts from these mice were significantly attenuated. Finally, we tested whether pregnancy-associated sex hormones could induce hypertrophy and alter signaling pathways in isolated neonatal rat ventricular myocytes (NRVMs). In fact, progesterone, but not estradiol treatment increased NRVM cell size via phosphorylation of ERK1/2. Inhibition of MEK1 effectively blocked progesterone-induced cellular hypertrophy. Taken together, our study demonstrates that pregnancy-induced cardiac hypertrophy is mediated by activation of Akt and ERK1/2 pathways.
Figures
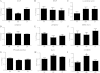

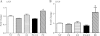
References
-
- Barnard RJ, Duncan HW, Baldwin KM, Grimditch G, Buckberg GD. Effects of intensive exercise training on myocardial performance and coronary blood flow. J Appl Physiol 49: 444–449, 1980 - PubMed
-
- Bocalini D, Carvalho E, de Sousa A, Levy R, Tucci P. Exercise training-induced enhancement in myocardial mechanics is lost after 2 weeks of detraining in rats. Eur J Appl Physiol 109: 909–914, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

