Hsp70 protein positively regulates rabies virus infection
- PMID: 22345440
- PMCID: PMC3347396
- DOI: 10.1128/JVI.06501-11
Hsp70 protein positively regulates rabies virus infection
Abstract
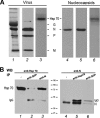
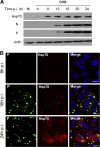
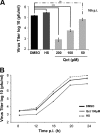
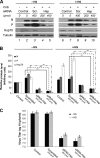
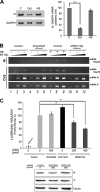
The Hsp70 chaperone plays a central role in multiple processes within cells, including protein translation, folding, intracellular trafficking, and degradation. This protein is implicated in the replication of numerous viruses. We have shown that rabies virus infection induced the cellular expression of Hsp70, which accumulated in Negri body-like structures, where viral transcription and replication take place. In addition, Hsp70 is present in both nucleocapsids purified from infected cells and in purified virions. Hsp70 has been shown to interact with the nucleoprotein N. The downregulation of Hsp70, using specific chaperone inhibitors, such as quercetin or RNA interference, resulted in a significant decrease of the amount of viral mRNAs, viral proteins, and virus particles. These results indicate that Hsp70 has a proviral function during rabies virus infection and suggest that Hsp70 is involved in at least one stage(s) of the viral life cycle, such as viral transcription, translation, and/or production. The mechanism by which Hsp70 controls viral infection will be discussed.
Figures
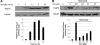

References
-
- Albertini AA, Ruigrok RW, Blondel D. 2011. Rabies virus transcription and replication. Adv. Virus Res. 79:1–22 - PubMed
-
- Brown G, et al. 2005. Evidence for an association between heat shock protein 70 and the respiratory syncytial virus polymerase complex within lipid-raft membranes during virus infection. Virology 338:69–80 - PubMed
-
- Bukau B, Deuerling E, Pfund C, Craig EA. 2000. Getting newly synthesized proteins into shape. Cell 101:119–122 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

