Spike protein VP8* of human rotavirus recognizes histo-blood group antigens in a type-specific manner
- PMID: 22345472
- PMCID: PMC3347384
- DOI: 10.1128/JVI.05507-11
Spike protein VP8* of human rotavirus recognizes histo-blood group antigens in a type-specific manner
Abstract
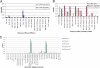
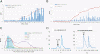
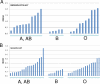
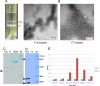
Rotaviruses (RVs), an important cause of severe diarrhea in children, have been found to recognize sialic acid as receptors for host cell attachment. While a few animal RVs (of P[1], P[2], P[3], and P[7]) are sialidase sensitive, human RVs and the majority of animal RVs are sialidase insensitive. In this study, we demonstrated that the surface spike protein VP8* of the major P genotypes of human RVs interacts with the secretor histo-blood group antigens (HBGAs). Strains of the P[4] and P[8] genotypes shared reactivity with the common antigens of Lewis b (Le(b)) and H type 1, while strains of the P[6] genotype bound the H type 1 antigen only. The bindings between recombinant VP8* and human saliva, milk, or synthetic HBGA oligosaccharides were demonstrated, which was confirmed by blockade of the bindings by monoclonal antibodies (MAbs) specific to Le(b) and/or H type 1. In addition, specific binding activities were observed when triple-layered particles of a P[8] (Wa) RV were tested. Our results suggest that the spike protein VP8* of RVs is involved in the recognition of human HBGAs that may function as ligands or receptors for RV attachment to host cells.
Figures




References
-
- Arias CF, et al. 2002. Molecular biology of rotavirus cell entry. Arch. Med. Res. 33:356–361 - PubMed
-
- Bahl R, et al. 2005. Incidence of severe rotavirus diarrhea in New Delhi, India, and G and P types of the infecting rotavirus strains. J. Infect. Dis. 192(Suppl. 1):S114–S119 - PubMed
-
- Beards G, Graham C. 1995. Temporal distribution of rotavirus G-serotypes in the West Midlands region of the United Kingdom, 1983–1994. J. Diarrhoeal Dis. Res. 13:235–237 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

