FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR
- PMID: 22345708
- PMCID: PMC3329390
- DOI: 10.1194/jlr.M025403
FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR
Abstract
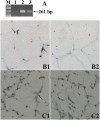
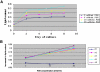
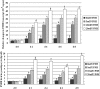
Transcripts and protein for follicle-stimulating hormone receptor (FSHR) were demonstrated in abdominal adipose tissue of female chickens. There was no expression of the Fsh gene, but FSH and FSHR colocalized, suggesting that FSH was receptor bound. Partial correlations indicted that changes in abdominal fat (AF) content were most directly correlated with Fshr mRNA expression, and the latter was directly correlated with tissue FSH content. These relationships were consistent with FSH inducing Fshr mRNA expression and with the finding that FSH influenced the accumulation of AF in chickens, a novel role for the hormone. Chicken preadipocytes responded linearly to doubling concentrations of FSH in Fshr mRNA expression and quantities of FSHR and lipid, without discernable effect on proliferation. Cells exposed to FSH more rapidly acquired adipocyte morphology. Treatment of young chickens with chicken FSH (4 mIU/day, subcutaneous, days 7-13) did not significantly decrease live weight but increased AF weight by 54.61%, AF as a percentage of live weight by 55.45%, and FSHR transcripts in AF by 222.15% (2 h after injection). In cells stimulated by FSH, genes related to lipid metabolism, including Rdh10, Dci, RarB, Lpl, Acsl3, and Dgat2, were expressed differentially, compared with no FSH. Several pathways of retinal and fatty acid metabolism, and peroxisome proliferator-activated receptor (PPAR) signaling changed. In conclusion, FSH stimulates lipid biosynthesis by upregulating Fshr mRNA expression in abdominal adipose tissue of chickens. Several genes involved in fatty acid and retinal metabolism and the PPAR signaling pathway mediate this novel function of FSH.
Figures



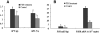
References
-
- Rannikki A. S., Zhang F. P., Huhtaniemi I. T. 1995. Ontogeny of follicle stimulating hormone receptor gene expression in the rat testis and ovary. Mol. Cell. Endocrinol. 107: 199–208 - PubMed
-
- Simoni M., Gromoli J., Niesclag E. 1997. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 18: 739–773 - PubMed
-
- Tilly J. L., LaPolt P. S., Hsuech A. J. W. 1992. Hormonal regulation of follicle stimulating hormone receptor messenger ribonucleic acid level in cultured rat granulose cells. Endocrinology. 130: 1296–1302 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

