A randomized controlled trial to compare the efficacy of bisphosphonates in the management of painful bone metastasis
- PMID: 22346045
- PMCID: PMC3276818
- DOI: 10.4103/0973-1075.92338
A randomized controlled trial to compare the efficacy of bisphosphonates in the management of painful bone metastasis
Abstract
Introduction: The prospective interventional single-institution randomized control study was carried out to compare the pain relieving efficacy among different bisphosphonates at the cost of incidence of skeletal-related events (SRE).
Materials and methods: During June 2008 and May 2011, 256 patients with painful bone metastasis in solid tumors with a pain score of at least 5 were randomized into three arms: zoledronic acid (4 mg, i.v.), ibandronate (6 mg, i.v.) and pamidronate (90 mg, i.v.). Radiation was given to all patients, either 800 cGy single fraction or 20 Gy in five fractions. The ANOVA test was used for analysis. The Pearson test was used to correlate pain scores with proportions of responders as statistical estimation of pain relief.
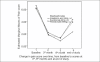
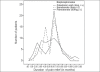
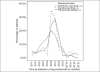
Results: With a mean baseline pain score of 6.5 ± 1.2, there was no difference in pain scores among the three treatment arms, assessed at 3 months and at the end of the study. However, the pain scores at 6 months were statistically reduced in zoledronic acid-receiving patients (1.5 ± 0.4) unlike the scores in patients receiving ibandronate (3.1 ± 0.5) and pamidronate (2.3 ± 0.4), with a P-value of 0.024. The response was statistically significant at 6 months (0.039) and at the end of the study (0.023), in favor of zoledronic acid. Pearson's correlation demonstrated a statistically significant positive correlation between pain scores and response rates. There were no statistical differences in the narcotic scores among the treatment arms during the study period. The overall duration of pain relief was not different in any of treatment arms. The time of detection of hypercalcemia was no different; however, the incidence of hypercalcemia was significantly less in the zoledronic acid arm (28.3%) against 44.6% and 50% in ibandronate and pamidronate arms, respectively, with a P-value of 0.041.
Conclusion: The use of bisphosphonates for 6 months or more results in a statistical significant improvement in bone pain, more so with zoledronic acid. Hypercalcemia, an SRE, was significantly less in the zoledronic acid arm.
Keywords: Bisphosphonates; Ibandronate; Painful bone metastasis; Pamidronate; Skeletal-related events; Zoledronic acid.
Conflict of interest statement
Figures


References
-
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of Zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–82. - PubMed
-
- Farrar JT. What is clinically meaningful: Outcome measures in pain clinical trials. Clin J Pain. 2000;16:106–12. - PubMed
-
- Hanks G. The crisis in health care cost in the United States: Some implications for radiation oncology. Int J Radiat Oncol Biol Phys. 1992;23:203–6. - PubMed
-
- Nielsen OS, Munro AJ, Tannock IF. Bone metastases: Pathophysiology and management policy. J Clin Oncol. 1991;9:509–24. - PubMed
LinkOut - more resources
Full Text Sources

