Stevens-johnson syndrome induced by vandetanib
- PMID: 22346274
- PMCID: PMC3276793
- DOI: 10.5021/ad.2011.23.S3.S343
Stevens-johnson syndrome induced by vandetanib
Abstract
Vandetanib is a once-daily oral anticancer drug that selectively inhibits key signaling pathways in cancer by targeting vascular endothelial growth factor receptors, epidermal growth factor receptors tyrosine kinase, and rearranged during transfection-dependent tumor cell proliferation and survival. The most frequently reported adverse events attributed to vandetanib include diarrhea, elevated aminotransferase, asymptomatic corrected QT interval prolongation, and hypertension. Though a number of randomized, doubleblind studies, including cutaneous adverse events attributed to vandetanib, have been reported along with these general symptoms, no case of Stevens-Johnson syndrome (SJS) has been reported. This paper demonstrates a case of SJS induced by vandetanib.
Keywords: Non-small-cell lung carcinoma; Stevens-Johnson syndrome; Vandetanib.
Figures
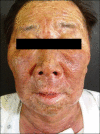
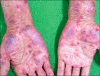

References
-
- Horti J, Widmark A, Stenzl A, Federico MH, Abratt RP, Sanders N, et al. A randomized, double-blind, placebocontrolled phase II study of vandetanib plus docetaxel/prednisolone in patients with hormone-refractory prostate cancer. Cancer Biother Radiopharm. 2009;24:175–180. - PubMed
-
- Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schröder W, Roujeau JC SCAR Study Group. Severe Cutaneous Adverse Reactions. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol. 2002;138:1019–1024. - PubMed
-
- Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–96. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

