Optical microspherical resonators for biomedical sensing
- PMID: 22346603
- PMCID: PMC3274111
- DOI: 10.3390/s110100785
Optical microspherical resonators for biomedical sensing
Abstract
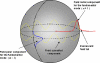
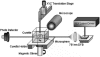
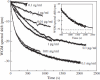
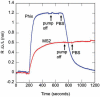
Optical resonators play an ubiquitous role in modern optics. A particular class of optical resonators is constituted by spherical dielectric structures, where optical rays are total internal reflected. Due to minimal reflection losses and to potentially very low material absorption, these guided modes, known as whispering gallery modes, can confer the resonator an exceptionally high quality factor Q, leading to high energy density, narrow resonant-wavelength lines and a lengthy cavity ringdown. These attractive characteristics make these miniaturized optical resonators especially suited as laser cavities and resonant filters, but also as very sensitive sensors. First, a brief analysis is presented of the characteristics of microspherical resonators, of their fabrication methods, and of the light coupling techniques. Then, we attempt to overview some of the recent advances in the development of microspherical biosensors, underlining a number of important applications in the biomedical field.
Keywords: biomedical sensors; microspheres; optical resonators; whispering gallery modes.
Figures




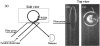




Similar articles
-
Biosensing by WGM Microspherical Resonators.Sensors (Basel). 2016 Jun 17;16(6):905. doi: 10.3390/s16060905. Sensors (Basel). 2016. PMID: 27322282 Free PMC article. Review.
-
Stimulated Stokes and Antistokes Raman Scattering in Microspherical Whispering Gallery Mode Resonators.J Vis Exp. 2016 Apr 4;(110):e53938. doi: 10.3791/53938. J Vis Exp. 2016. PMID: 27078752 Free PMC article.
-
From Whispering Gallery Mode Resonators to Biochemical Sensors.ACS Sens. 2023 Jul 28;8(7):2440-2470. doi: 10.1021/acssensors.2c02876. Epub 2023 Jun 30. ACS Sens. 2023. PMID: 37390481 Review.
-
Batch Fabrication of High-Quality Infrared Chalcogenide Microsphere Resonators.Small. 2021 May;17(20):e2100140. doi: 10.1002/smll.202100140. Epub 2021 Apr 2. Small. 2021. PMID: 33811462
-
Unveiling the Coupling of Single Metallic Nanoparticles to Whispering-Gallery Microcavities.Nano Lett. 2022 Jan 12;22(1):319-327. doi: 10.1021/acs.nanolett.1c03826. Epub 2021 Dec 15. Nano Lett. 2022. PMID: 34907775
Cited by
-
Aptasensors Based on Whispering Gallery Mode Resonators.Biosensors (Basel). 2016 Jul 16;6(3):28. doi: 10.3390/bios6030028. Biosensors (Basel). 2016. PMID: 27438861 Free PMC article. Review.
-
Performance of Eudragit coated whispering gallery mode resonator-based immunosensors.Sensors (Basel). 2012 Oct 30;12(11):14604-11. doi: 10.3390/s121114604. Sensors (Basel). 2012. PMID: 23202178 Free PMC article.
-
Spatial-Division Multiplexing Approach for Simultaneous Detection of Fiber-Optic Ball Resonator Sensors: Applications for Refractometers and Biosensors.Biosensors (Basel). 2022 Nov 11;12(11):1007. doi: 10.3390/bios12111007. Biosensors (Basel). 2022. PMID: 36421126 Free PMC article.
-
Modulation techniques for biomedical implanted devices and their challenges.Sensors (Basel). 2012;12(1):297-319. doi: 10.3390/s120100297. Epub 2011 Dec 28. Sensors (Basel). 2012. PMID: 22368470 Free PMC article. Review.
-
Optical Ring Resonators: A Platform for Biological Sensing Applications.J Med Signals Sens. 2017 Jul-Sep;7(3):185-191. J Med Signals Sens. 2017. PMID: 28840120 Free PMC article.
References
-
- Luong J.H., Male K.B., Glennon J.D. Biosensor technology: Technology push versus market pull. Biotechnol. Adv. 2008;26:492–500. - PubMed
-
- Shao Y., Wang J., Wu H., Liu J., Aksay I.A., Lina Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis. 2010;22:1027–1036.
-
- Hoa X.D., Kirk A.G., Tabrizian M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron. 2007;23:151–160. - PubMed
-
- Leung A., Shankar P.M., Mutharasan R. A review of fiber-optic biosensors. Sens. Actuat B. 2007;125:688–703.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

