Bio-sensing of cadmium(II) ions using Staphylococcus aureus
- PMID: 22346664
- PMCID: PMC3274306
- DOI: 10.3390/s111110638
Bio-sensing of cadmium(II) ions using Staphylococcus aureus
Abstract
Cadmium, as a hazardous pollutant commonly present in the living environment, represents an important risk to human health due to its undesirable effects (oxidative stress, changes in activities of many enzymes, interactions with biomolecules including DNA and RNA) and consequent potential risk, making its detection very important. New and unique technological and biotechnological approaches for solving this problems are intensely sought. In this study, we used the commonly occurring potential pathogenic microorganism Staphylococcus aureus for the determination of markers which could be used for sensing of cadmium(II) ions. We were focused on monitoring the effects of different cadmium(II) ion concentrations (0, 1.25, 2.5, 5, 10, 15, 25 and 50 μg mL(-1)) on the growth and energetic metabolism of Staphylococcus aureus. Highly significant changes have been detected in the metabolism of thiol compounds-specifically the protein metallothionein (0.79-26.82 mmol/mg of protein), the enzyme glutathione S-transferase (190-5,827 μmol/min/mg of protein), and sulfhydryl groups (9.6-274.3 μmol cysteine/mg of protein). The ratio of reduced and oxidized glutathione indicated marked oxidative stress. In addition, dramatic changes in urease activity, which is connected with resistance of bacteria, were determined. Further, the effects of cadmium(II) ions on the metabolic pathways of arginine, β-glucosidase, phosphatase, N-acetyl β-d-glucosamine, sucrose, trehalose, mannitol, maltose, lactose, fructose and total proteins were demonstrated. A metabolomic profile of Staphylococcus aureus under cadmium(II) ion treatment conditions was completed seeking data about the possibility of cadmium(II) ion accumulation in cells. The results demonstrate potential in the application of microorganisms as modern biosensor systems based on biological components.
Keywords: Brdicka reaction; Staphylococcus aureus; biosensor; cadmium; electrochemistry; high performance liquid chromatography with electrochemical detection; metabolic activity; metabolome; microbiome; spectrophotometry; voltammetry.
Figures


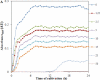





References
-
- Han F.X.X., Banin A., Su Y., Monts D.L., Plodinec M.J., Kingery W.L., Triplett G.E. Industrial age anthropogenic inputs of heavy metals into the pedosphere. Naturwissenschaften. 2002;89:497–504. - PubMed
-
- UNEP Lead (pb) and Cadmium (Cd) at the 26th Session of the Governing Council Nairobi 21 to 24 February 2011. Available online: http://www.unep.org/hazardoussubstances/LeadCadmium/ScientificReviews/Pr... (accessed on 16 September 2011).
-
- Agency for Toxic Substance and Disease Registry (ATSDR), US Toxicological Profile for Cadmium. Department of Health and Humans Services, Public Health Service, Centers for Disease Control; Atlanta, GA, USA: Available online: http://www.atsdr.cdc.gov/toxprofiles/tp5.pdf (accessed on 16 September 2011).
-
- Garrett R.G. Natural sources of metals to the environment. Hum. Ecol. Risk Assess. 2000;6:945–963.
-
- Johansson C., Norman M., Burman L. Road traffic emission factors for heavy metals. Atmos. Environ. 2009;43:4681–4688.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

