Level-Set Variational Implicit-Solvent Modeling of Biomolecules with the Coulomb-Field Approximation
- PMID: 22346739
- PMCID: PMC3278970
- DOI: 10.1021/ct200647j
Level-Set Variational Implicit-Solvent Modeling of Biomolecules with the Coulomb-Field Approximation
Abstract
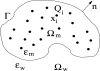
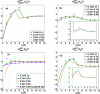
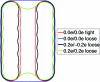
Central in the variational implicit-solvent model (VISM) [Dzubiella, Swanson, and McCammon Phys. Rev. Lett.2006, 96, 087802 and J. Chem. Phys.2006, 124, 084905] of molecular solvation is a mean-field free-energy functional of all possible solute-solvent interfaces or dielectric boundaries. Such a functional can be minimized numerically by a level-set method to determine stable equilibrium conformations and solvation free energies. Applications to nonpolar systems have shown that the level-set VISM is efficient and leads to qualitatively and often quantitatively correct results. In particular, it is capable of capturing capillary evaporation in hydrophobic confinement and corresponding multiple equilibrium states as found in molecular dynamics (MD) simulations. In this work, we introduce into the VISM the Coulomb-field approximation of the electrostatic free energy. Such an approximation is a volume integral over an arbitrary shaped solvent region, requiring no solutions to any partial differential equations. With this approximation, we obtain the effective boundary force and use it as the "normal velocity" in the level-set relaxation. We test the new approach by calculating solvation free energies and potentials of mean force for small and large molecules, including the two-domain protein BphC. Our results reveal the importance of coupling polar and nonpolar interactions in the underlying molecular systems. In particular, dehydration near the domain interface of BphC subunits is found to be highly sensitive to local electrostatic potentials as seen in previous MD simulations. This is a first step toward capturing the complex protein dehydration process by an implicit-solvent approach.
Figures
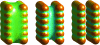



Similar articles
-
A self-consistent phase-field approach to implicit solvation of charged molecules with Poisson-Boltzmann electrostatics.J Chem Phys. 2015 Dec 28;143(24):243110. doi: 10.1063/1.4932336. J Chem Phys. 2015. PMID: 26723595 Free PMC article.
-
Coupling the Level-Set Method with Molecular Mechanics for Variational Implicit Solvation of Nonpolar Molecules.J Chem Theory Comput. 2009 Feb 10;5(2):257-266. doi: 10.1021/ct800297d. J Chem Theory Comput. 2009. PMID: 20150952 Free PMC article.
-
Application of the level-set method to the implicit solvation of nonpolar molecules.J Chem Phys. 2007 Aug 28;127(8):084503. doi: 10.1063/1.2757169. J Chem Phys. 2007. PMID: 17764265
-
Tailoring the Variational Implicit Solvent Method for New Challenges: Biomolecular Recognition and Assembly.Front Mol Biosci. 2018 Feb 12;5:13. doi: 10.3389/fmolb.2018.00013. eCollection 2018. Front Mol Biosci. 2018. PMID: 29484300 Free PMC article. Review.
-
Implicit modeling of nonpolar solvation for simulating protein folding and conformational transitions.Phys Chem Chem Phys. 2008 Jan 28;10(4):471-81. doi: 10.1039/b714141f. Epub 2007 Nov 14. Phys Chem Chem Phys. 2008. PMID: 18183310 Review.
Cited by
-
DIFFUSED SOLUTE-SOLVENT INTERFACE WITH POISSON-BOLTZMANN ELECTROSTATICS: FREE-ENERGY VARIATION AND SHARP-INTERFACE LIMIT.SIAM J Appl Math. 2015;75(5):2072-2092. doi: 10.1137/15M100701X. Epub 2015 Sep 15. SIAM J Appl Math. 2015. PMID: 26877556 Free PMC article.
-
Phase-field approach to implicit solvation of biomolecules with Coulomb-field approximation.J Chem Phys. 2013 Jul 14;139(2):024111. doi: 10.1063/1.4812839. J Chem Phys. 2013. PMID: 23862933 Free PMC article.
-
Evaluation of Hydration Free Energy by Level-Set Variational Implicit-Solvent Model with Coulomb-Field Approximation.J Chem Theory Comput. 2013 Mar 12;9(3):1778-1787. doi: 10.1021/ct301087w. Epub 2013 Jan 29. J Chem Theory Comput. 2013. PMID: 23505345 Free PMC article.
-
Variational implicit-solvent predictions of the dry-wet transition pathways for ligand-receptor binding and unbinding kinetics.Proc Natl Acad Sci U S A. 2019 Jul 23;116(30):14989-14994. doi: 10.1073/pnas.1902719116. Epub 2019 Jul 3. Proc Natl Acad Sci U S A. 2019. PMID: 31270236 Free PMC article.
-
Identification of protein-ligand binding sites by the level-set variational implicit-solvent approach.J Chem Theory Comput. 2015 Feb 10;11(2):753-65. doi: 10.1021/ct500867u. J Chem Theory Comput. 2015. PMID: 25941465 Free PMC article.
