Subcellular location of PKA controls striatal plasticity: stochastic simulations in spiny dendrites
- PMID: 22346744
- PMCID: PMC3276550
- DOI: 10.1371/journal.pcbi.1002383
Subcellular location of PKA controls striatal plasticity: stochastic simulations in spiny dendrites
Abstract
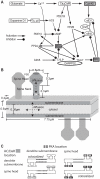
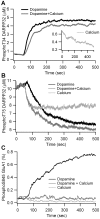
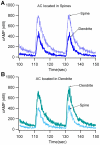
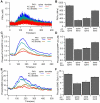
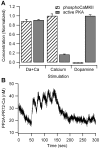
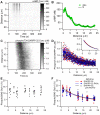
Dopamine release in the striatum has been implicated in various forms of reward dependent learning. Dopamine leads to production of cAMP and activation of protein kinase A (PKA), which are involved in striatal synaptic plasticity and learning. PKA and its protein targets are not diffusely located throughout the neuron, but are confined to various subcellular compartments by anchoring molecules such as A-Kinase Anchoring Proteins (AKAPs). Experiments have shown that blocking the interaction of PKA with AKAPs disrupts its subcellular location and prevents LTP in the hippocampus and striatum; however, these experiments have not revealed whether the critical function of anchoring is to locate PKA near the cAMP that activates it or near its targets, such as AMPA receptors located in the post-synaptic density. We have developed a large scale stochastic reaction-diffusion model of signaling pathways in a medium spiny projection neuron dendrite with spines, based on published biochemical measurements, to investigate this question and to evaluate whether dopamine signaling exhibits spatial specificity post-synaptically. The model was stimulated with dopamine pulses mimicking those recorded in response to reward. Simulations show that PKA colocalization with adenylate cyclase, either in the spine head or in the dendrite, leads to greater phosphorylation of DARPP-32 Thr34 and AMPA receptor GluA1 Ser845 than when PKA is anchored away from adenylate cyclase. Simulations further demonstrate that though cAMP exhibits a strong spatial gradient, diffusible DARPP-32 facilitates the spread of PKA activity, suggesting that additional inactivation mechanisms are required to produce spatial specificity of PKA activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Hiroi N, Fienberg AA, Haile CN, Alburges M, Hanson GR, et al. Neuronal and behavioural abnormalities in striatal function in DARPP-32-mutant mice. Eur J Neurosci. 1999;11:1114–1118. - PubMed
-
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. - PubMed
-
- Cromwell HC, Hassani OK, Schultz W. Relative reward processing in primate striatum. Exp Brain Res. 2005;162:520–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

