A regulatory network for coordinated flower maturation
- PMID: 22346763
- PMCID: PMC3276552
- DOI: 10.1371/journal.pgen.1002506
A regulatory network for coordinated flower maturation
Abstract
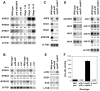
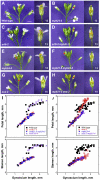
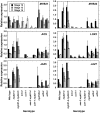
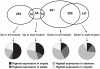
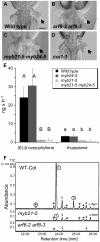
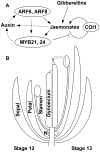
For self-pollinating plants to reproduce, male and female organ development must be coordinated as flowers mature. The Arabidopsis transcription factors AUXIN RESPONSE FACTOR 6 (ARF6) and ARF8 regulate this complex process by promoting petal expansion, stamen filament elongation, anther dehiscence, and gynoecium maturation, thereby ensuring that pollen released from the anthers is deposited on the stigma of a receptive gynoecium. ARF6 and ARF8 induce jasmonate production, which in turn triggers expression of MYB21 and MYB24, encoding R2R3 MYB transcription factors that promote petal and stamen growth. To understand the dynamics of this flower maturation regulatory network, we have characterized morphological, chemical, and global gene expression phenotypes of arf, myb, and jasmonate pathway mutant flowers. We found that MYB21 and MYB24 promoted not only petal and stamen development but also gynoecium growth. As well as regulating reproductive competence, both the ARF and MYB factors promoted nectary development or function and volatile sesquiterpene production, which may attract insect pollinators and/or repel pathogens. Mutants lacking jasmonate synthesis or response had decreased MYB21 expression and stamen and petal growth at the stage when flowers normally open, but had increased MYB21 expression in petals of older flowers, resulting in renewed and persistent petal expansion at later stages. Both auxin response and jasmonate synthesis promoted positive feedbacks that may ensure rapid petal and stamen growth as flowers open. MYB21 also fed back negatively on expression of jasmonate biosynthesis pathway genes to decrease flower jasmonate level, which correlated with termination of growth after flowers have opened. These dynamic feedbacks may promote timely, coordinated, and transient growth of flower organs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Nagpal P, Ellis CM, Weber H, Ploense SE, Barkawi LS, et al. Auxin Response Factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development. 2005;132:4107–4118. - PubMed
-
- Tabata R, Ikezaki M, Fujibe T, Aida M, Tian CE, et al. Arabidopsis AUXIN RESPONSE FACTOR6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class 1 KNOX genes. Plant Cell Physiol. 2010;51:164–175. - PubMed
-
- Wu M-F, Tian Q, Reed JW. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression and regulates both female and male reproduction. Development. 2006;133:4211–4218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

