1,1,4,4-Tetra-tert-butyl-1,4-dichloro-2,2,3,3-tetra-phenyl-tetra-silane
- PMID: 22347038
- PMCID: PMC3275182
- DOI: 10.1107/S1600536812000669
1,1,4,4-Tetra-tert-butyl-1,4-dichloro-2,2,3,3-tetra-phenyl-tetra-silane
Abstract
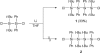
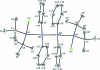
The title compound, C(40)H(56)Cl(2)Si(4), was synthesized by the coupling of 1,1-di-tert-butyl-1,2-dichloro-2,2-diphenyl-disilane with lithium. The asymmetric unit contains one half-mol-ecule, which is completed by an inversion centre. In the mol-ecule, the tetra-silane skeleton adopts a perfect anti conformation and the Si-Si bonds [2.4355 (5) and 2.4328 (7) Å] are longer than the standard Si-Si bond length (2.34 Å). The Si-Si-Si angle [116.09 (2)°] is larger than the tetra-hedral bond angle (109.5°). These long bond lengths and the wide angle are favorable for reducing the steric hindrance among the tert-butyl and the phenyl groups. The dihedral angle between the phenyl rings in the asymmetric unit is 37.36 (8)°.
Figures

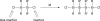
References
-
- Baumeister, U., Schenzel, K., Zink, R. & Hassler, K. (1997). J. Organomet. Chem. 543, 117–124.
-
- Burkhard, C. A. (1949). J. Am. Chem. Soc. 71, 963–964.
-
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., De Caro, L., Giacovazzo, C., Polidori, G. & Spagna, R. (2005). J. Appl. Cryst. 38, 381–388.
-
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
-
- Fukazawa, A., Tsuji, H. & Tamao, K. (2006). J. Am. Chem. Soc. 128, 6800–6801. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
