2-Oxo-2H-chromen-4-yl 4-tert-butyl-benzoate
- PMID: 22347133
- PMCID: PMC3275277
- DOI: 10.1107/S160053681200298X
2-Oxo-2H-chromen-4-yl 4-tert-butyl-benzoate
Abstract
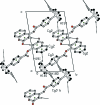
In the title mol-ecule, C(20)H(18)O(4), the three methyl groups of the tert-butyl substituent show rotational disorder. Each methyl group is split over three positions, with refined site-occupation factors of 0.711 (4), 0.146 (3) and 0.144 (4). The benzene ring of the benzoate group is oriented at a dihedral angle of 60.70 (7)° with respect to the planar chromene ring [maximum deviation = 0.046 (2) Å]. The crystal structure features centrosymmetric R(2) (2)(8) dimers formed via C-H⋯O inter-actions, and these dimeric aggregates are connected by C-H⋯π inter-actions.
Figures


References
-
- Abd Elhafez, O. M., El Khrisy, A. M., Badria, F. & Fathy, A. M. (2003). Arch. Pharm. Res. 26, 686–696. - PubMed
-
- Basanagouda, M., Kulkarni, M. V., Sharma, D., Gupta, V. K., Pranesha, Sandhyarani, P. & Rasal, V. P. (2009). J. Chem. Sci. 121, 485–495.
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
-
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., De Caro, L., Giacovazzo, C., Polidori, G. & Spagna, R. (2005). J. Appl. Cryst. 38, 381–388.
-
- Emmanuel-Giota, A. A., Fylaktakidou, K. C., Hadjipavlou-Litina, D. J., Litinas, K. E. & Nicolaides, D. N. (2001). J. Heterocycl. Chem. 38, 717–722.
LinkOut - more resources
Full Text Sources
