Coordinating Gene Expression and Axon Assembly to Control Axon Growth: Potential Role of GSK3 Signaling
- PMID: 22347166
- PMCID: PMC3272657
- DOI: 10.3389/fnmol.2012.00003
Coordinating Gene Expression and Axon Assembly to Control Axon Growth: Potential Role of GSK3 Signaling
Abstract
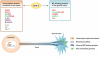
Axon growth requires the coordinated regulation of gene expression in the neuronal soma, local protein translation in the axon, anterograde transport of synthesized raw materials along the axon, and assembly of cytoskeleton and membranes in the nerve growth cone. Glycogen synthase kinase 3 (GSK3) signaling has recently been shown to play key roles in the regulation of axonal transport and cytoskeletal assembly during axon growth. GSK3 signaling is also known to regulate gene expression via controlling the functions of many transcription factors, suggesting that GSK3 may be an important regulator of gene transcription supporting axon growth. We review signaling pathways that control local axon assembly at the growth cone and gene expression in the soma during developmental or regenerative axon growth and discuss the potential involvement of GSK3 signaling in these processes, with a particular focus on how GSK3 signaling modulates the function of axon growth-associated transcription factors.
Keywords: axon growth; axon regeneration; glycogen synthase kinase 3; transcription factor.
Figures
References
-
- Arthur J. S., Fong A. L., Dwyer J. M., Davare M., Reese E., Obrietan K., Impey S. (2004). Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J. Neurosci. 24, 4324–4332 10.1523/JNEUROSCI.5227-03.2004 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

