Energetics based spike generation of a single neuron: simulation results and analysis
- PMID: 22347180
- PMCID: PMC3269776
- DOI: 10.3389/fnene.2012.00002
Energetics based spike generation of a single neuron: simulation results and analysis
Abstract
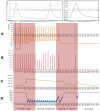
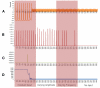
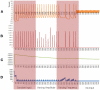
Existing current based models that capture spike activity, though useful in studying information processing capabilities of neurons, fail to throw light on their internal functioning. It is imperative to develop a model that captures the spike train of a neuron as a function of its intracellular parameters for non-invasive diagnosis of diseased neurons. This is the first ever article to present such an integrated model that quantifies the inter-dependency between spike activity and intracellular energetics. The generated spike trains from our integrated model will throw greater light on the intracellular energetics than existing current models. Now, an abnormality in the spike of a diseased neuron can be linked and hence effectively analyzed at the energetics level. The spectral analysis of the generated spike trains in a time-frequency domain will help identify abnormalities in the internals of a neuron. As a case study, the parameters of our model are tuned for Alzheimer's disease and its resultant spike trains are studied and presented. This massive initiative ultimately aims to encompass the entire molecular signaling pathways of the neuronal bioenergetics linking it to the voltage spike initiation and propagation; due to the lack of experimental data quantifying the inter dependencies among the parameters, the model at this stage adopts a particular level of functionality and is shown as an approach to study and perform disease modeling at the spike train and the mitochondrial bioenergetics level.
Keywords: ATP; Alzheimer’s disease; Krebs cycle; Petri nets; mitochondria; neuroenergetics; voltage spike; wavelet transformations.
Figures





Similar articles
-
Identification of time-varying neural dynamics from spike train data using multiwavelet basis functions.J Neurosci Methods. 2017 Feb 15;278:46-56. doi: 10.1016/j.jneumeth.2016.12.018. Epub 2017 Jan 4. J Neurosci Methods. 2017. PMID: 28062244
-
Information transmission in hippocampal CA1 neuron models in the presence of poisson shot noise: the case of periodic sub-threshold spike trains.Conf Proc IEEE Eng Med Biol Soc. 2006;2006:4196-9. doi: 10.1109/IEMBS.2006.260237. Conf Proc IEEE Eng Med Biol Soc. 2006. PMID: 17945831
-
Multineuron spike train analysis with R-convolution linear combination kernel.Neural Netw. 2018 Jun;102:67-77. doi: 10.1016/j.neunet.2018.02.013. Epub 2018 Mar 6. Neural Netw. 2018. PMID: 29544140
-
Nonlinear dynamic modeling of spike train transformations for hippocampal-cortical prostheses.IEEE Trans Biomed Eng. 2007 Jun;54(6 Pt 1):1053-66. doi: 10.1109/TBME.2007.891948. IEEE Trans Biomed Eng. 2007. PMID: 17554824
-
Statistical models for neural encoding, decoding, and optimal stimulus design.Prog Brain Res. 2007;165:493-507. doi: 10.1016/S0079-6123(06)65031-0. Prog Brain Res. 2007. PMID: 17925266 Review.
Cited by
-
Understanding how the brain ensures its energy supply.Front Neuroenergetics. 2012 Aug 20;4:9. doi: 10.3389/fnene.2012.00009. eCollection 2012. Front Neuroenergetics. 2012. PMID: 22933995 Free PMC article. No abstract available.
-
Metabolic studies in brain slices - past, present, and future.Front Pharmacol. 2012 Mar 9;3:26. doi: 10.3389/fphar.2012.00026. eCollection 2012. Front Pharmacol. 2012. PMID: 22408619 Free PMC article. No abstract available.
-
A computational model of motor neuron degeneration.Neuron. 2014 Aug 20;83(4):975-88. doi: 10.1016/j.neuron.2014.07.001. Epub 2014 Jul 31. Neuron. 2014. PMID: 25088365 Free PMC article.
References
-
- Attwell D., Laughlin S. B. (2001). An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21, 1133–1145 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

