Inherent structural disorder and dimerisation of murine norovirus NS1-2 protein
- PMID: 22347381
- PMCID: PMC3274520
- DOI: 10.1371/journal.pone.0030534
Inherent structural disorder and dimerisation of murine norovirus NS1-2 protein
Abstract
Human noroviruses are highly infectious viruses that cause the majority of acute, non-bacterial epidemic gastroenteritis cases worldwide. The first open reading frame of the norovirus RNA genome encodes for a polyprotein that is cleaved by the viral protease into six non-structural proteins. The first non-structural protein, NS1-2, lacks any significant sequence similarity to other viral or cellular proteins and limited information is available about the function and biophysical characteristics of this protein. Bioinformatic analyses identified an inherently disordered region (residues 1-142) in the highly divergent N-terminal region of the norovirus NS1-2 protein. Expression and purification of the NS1-2 protein of Murine norovirus confirmed these predictions by identifying several features typical of an inherently disordered protein. These were a biased amino acid composition with enrichment in the disorder promoting residues serine and proline, a lack of predicted secondary structure, a hydrophilic nature, an aberrant electrophoretic migration, an increased Stokes radius similar to that predicted for a protein from the pre-molten globule family, a high sensitivity to thermolysin proteolysis and a circular dichroism spectrum typical of an inherently disordered protein. The purification of the NS1-2 protein also identified the presence of an NS1-2 dimer in Escherichia coli and transfected HEK293T cells. Inherent disorder provides significant advantages including structural flexibility and the ability to bind to numerous targets allowing a single protein to have multiple functions. These advantages combined with the potential functional advantages of multimerisation suggest a multi-functional role for the NS1-2 protein.
Conflict of interest statement
Figures
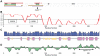


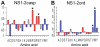
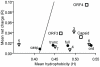
 . Proteins (or regions of proteins) shown to the left of the boundary line are predicted to be intrinsically disordered. Proteins to the right of the boundary line are predicted to be structured. NS1-2 regions (•); N-terminal caspase cleavage product (casp), truncated NS1-2 protein (trunc), full-length NS1-2 (full), middle ordered region (ord). The other MNV-1 non-structural proteins (∇) are numbered 2–7. Structural proteins are indicated by □.
. Proteins (or regions of proteins) shown to the left of the boundary line are predicted to be intrinsically disordered. Proteins to the right of the boundary line are predicted to be structured. NS1-2 regions (•); N-terminal caspase cleavage product (casp), truncated NS1-2 protein (trunc), full-length NS1-2 (full), middle ordered region (ord). The other MNV-1 non-structural proteins (∇) are numbered 2–7. Structural proteins are indicated by □.


References
-
- Fankhauser RL, Monroe SS, Noel JS, Humphrey CD, Bresee JS, et al. Epidemiologic and molecular trends of Norwalk-like viruses associated with outbreaks of gastroenteritis in the United States. J Infect Dis. 2002;186:1–7. - PubMed
-
- Kirkwood CD, Streitberg R. Calicivirus shedding in children after recovery from diarrhoeal disease. J Clin Virol. 2008;43:346–348. - PubMed
-
- Zintz C, Bok K, Parada E, Barnes-Eley M, Berke T, et al. Prevalence and genetic characterization of caliciviruses among children hospitalized for acute gastroenteritis in the United States. Infect Genet Evol. 2005;5:281–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

