The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke
- PMID: 22347410
- PMCID: PMC3275571
- DOI: 10.1371/journal.pone.0030892
The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke
Abstract
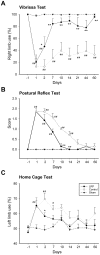
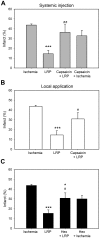
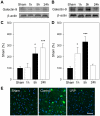
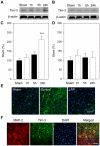
We recently demonstrated that limb remote preconditioning (LRP) protects against focal ischemia measured 2 days post-stroke. Here, we studied whether LRP provides long-term protection and improves neurological function. We also investigated whether LRP transmits its protective signaling via the afferent nerve pathways from the preconditioned limb to the ischemic brain and whether inflammatory factors are involved in LRP, including the novel galectin-9/Tim-3 inflammatory cell signaling pathway, which induces cell death in lymphocytes. LRP in the left hind femoral artery was performed immediately before stroke. LRP reduced brain injury size both at 2 days and 60 days post-stroke and improved behavioral outcomes for up to 2 months. The sensory nerve inhibitors capsaicin and hexamethonium, a ganglion blocker, abolished the protective effects of LRP. In addition, LRP inhibited edema formation and blood-brain barrier (BBB) permeability measured 2 days post-stroke. Western blot and immunostaining analysis showed that LRP inhibited protein expression of both galectin-9 and T-cell immunoglobulin domain and mucin domain 3 (Tim-3), which were increased after stroke. In addition, LRP decreased iNOS and nitrotyrosine protein expression after stroke. In conclusion, LRP executes long-term protective effects against stroke and may block brain injury by inhibiting activities of the galectin-9/Tim-3 pathway, iNOS, and nitrotyrosine.
Conflict of interest statement
Figures


References
-
- Zhao HG, Li WB, Li QJ, Chen XL, Liu HQ, et al. Limb ischemic preconditioning attenuates apoptosis of pyramidal neurons in the CA1 hippocampus induced by cerebral ischemia-reperfusion in rats. Sheng Li Xue Bao. 2004;56:407–412. - PubMed
-
- Dave KR, Saul I, Prado R, Busto R, Perez-Pinzon MA. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neurosci Lett. 2006;404:170–175. - PubMed
-
- Jensen HA, Loukogeorgakis S, Yannopoulos F, Rimpilainen E, Petzold A, et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123:714–721. - PubMed
-
- Malhotra S, Naggar I, Stewart M, Rosenbaum DM. Neurogenic pathway mediated remote preconditioning protects the brain from transient focal ischemic injury. Brain Res. 2011;1386:184–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

