Allotransplanted neurons used to repair peripheral nerve injury do not elicit overt immunogenicity
- PMID: 22347502
- PMCID: PMC3276507
- DOI: 10.1371/journal.pone.0031675
Allotransplanted neurons used to repair peripheral nerve injury do not elicit overt immunogenicity
Abstract
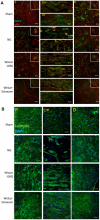
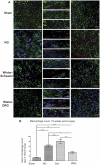
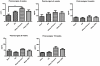
A major problem hindering the development of autograft alternatives for repairing peripheral nerve injuries is immunogenicity. We have previously shown successful regeneration in transected rat sciatic nerves using conduits filled with allogeneic dorsal root ganglion (DRG) cells without any immunosuppression. In this study, we re-examined the immunogenicity of our DRG neuron implanted conduits as a potential strategy to overcome transplant rejection. A biodegradable NeuraGen® tube was infused with pure DRG neurons or Schwann cells cultured from a rat strain differing from the host rats and used to repair 8 mm gaps in the sciatic nerve. We observed enhanced regeneration with allogeneic cells compared to empty conduits 16 weeks post-surgery, but morphological analyses suggest recovery comparable to the healthy nerves was not achieved. The degree of regeneration was indistinguishable between DRG and Schwann cell allografts although immunogenicity assessments revealed substantially increased presence of Interferon gamma (IFN-γ) in Schwann cell allografts compared to the DRG allografts by two weeks post-surgery. Macrophage infiltration of the regenerated nerve graft in the DRG group 16 weeks post-surgery was below the level of the empty conduit (0.56 fold change from NG; p<0.05) while the Schwann cell group revealed significantly higher counts (1.29 fold change from NG; p<0.001). Major histocompatibility complex I (MHC I) molecules were present in significantly increased levels in the DRG and Schwann cell allograft groups compared to the hollow NG conduit and the Sham healthy nerve. Our results confirmed previous studies that have reported Schwann cells as being immunogenic, likely due to MHC I expression. Nerve gap injuries are difficult to repair; our data suggest that DRG neurons are superior medium to implant inside conduit tubes due to reduced immunogenicity and represent a potential treatment strategy that could be preferable to the current gold standard of autologous nerve transplant.
Conflict of interest statement
Figures



Similar articles
-
Allotransplanted DRG neurons or Schwann cells affect functional recovery in a rodent model of sciatic nerve injury.Neurol Res. 2014 Nov;36(11):1020-1027. doi: 10.1179/1743132814Y.0000000386. Epub 2014 May 18. Neurol Res. 2014. PMID: 24836462 Free PMC article.
-
Oxidized galectin-1 stimulates the migration of Schwann cells from both proximal and distal stumps of transected nerves and promotes axonal regeneration after peripheral nerve injury.J Neuropathol Exp Neurol. 2003 Feb;62(2):162-72. doi: 10.1093/jnen/62.2.162. J Neuropathol Exp Neurol. 2003. PMID: 12578226
-
Enhancement of nerve regeneration through schwann cell-mediated healing in a 3D printed polyacrylonitrile conduit incorporating hydrogel and graphene quantum dots: a study on rat sciatic nerve injury model.Biomed Mater. 2023 Dec 21;19(1). doi: 10.1088/1748-605X/ad1576. Biomed Mater. 2023. PMID: 38091624
-
Advances in Large Gap Peripheral Nerve Injury Repair and Regeneration with Bridging Nerve Guidance Conduits.Macromol Biosci. 2023 Oct;23(10):e2300078. doi: 10.1002/mabi.202300078. Epub 2023 Jun 14. Macromol Biosci. 2023. PMID: 37235853 Review.
-
Optimizing Peripheral Nerve Regeneration: Surgical Techniques, Biomolecular and Regenerative Strategies-A Narrative Review.Int J Mol Sci. 2025 Apr 20;26(8):3895. doi: 10.3390/ijms26083895. Int J Mol Sci. 2025. PMID: 40332790 Free PMC article. Review.
Cited by
-
Typical and atypical properties of peripheral nerve allografts enable novel strategies to repair segmental-loss injuries.J Neuroinflammation. 2022 Feb 28;19(1):60. doi: 10.1186/s12974-022-02395-0. J Neuroinflammation. 2022. PMID: 35227261 Free PMC article. Review.
-
Electrical stimulation induces calcium-dependent neurite outgrowth and immediate early genes expressions of dorsal root ganglion neurons.Neurochem Res. 2014 Jan;39(1):129-41. doi: 10.1007/s11064-013-1197-7. Epub 2013 Nov 19. Neurochem Res. 2014. PMID: 24248860
-
Potential Mechanism of Neurite Outgrowth Enhanced by Electrical Stimulation: Involvement of MicroRNA-363-5p Targeting DCLK1 Expression in Rat.Neurochem Res. 2017 Feb;42(2):513-525. doi: 10.1007/s11064-016-2100-0. Epub 2016 Nov 30. Neurochem Res. 2017. PMID: 27900578
-
An allogeneic 'off the shelf' therapeutic strategy for peripheral nerve tissue engineering using clinical grade human neural stem cells.Sci Rep. 2018 Feb 13;8(1):2951. doi: 10.1038/s41598-018-20927-8. Sci Rep. 2018. PMID: 29440680 Free PMC article.
-
An update-tissue engineered nerve grafts for the repair of peripheral nerve injuries.Neural Regen Res. 2018 May;13(5):764-774. doi: 10.4103/1673-5374.232458. Neural Regen Res. 2018. PMID: 29862995 Free PMC article.
References
-
- Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–122. - PubMed
-
- Huang JH, Zaghloul K, Zager EL. Surgical management of brachial plexus region tumors. Surg Neurol. 2004;61:372–378. - PubMed
-
- Millesi H, Meissl G, Berger A. The interfascicular nerve-grafting of the median and ulnar nerves. J Bone Joint Surg Am. 1972;54:727–750. - PubMed
-
- Rodriguez FJ, Verdu E, Ceballos D, Navarro X. Nerve guides seeded with autologous schwann cells improve nerve regeneration. Exp Neurol. 2000;161:571–584. - PubMed
-
- Vert M, Li S, Garreau H. New insights on the degradation of bioresorbable polymeric devices based on lactic and glycolic acids. Clin Mater. 1992;10:3–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

