Inhibitors of the cellular trafficking of ricin
- PMID: 22347620
- PMCID: PMC3277095
- DOI: 10.3390/toxins4010015
Inhibitors of the cellular trafficking of ricin
Abstract
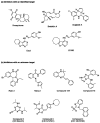
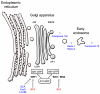
Throughout the last decade, efforts to identify and develop effective inhibitors of the ricin toxin have focused on targeting its N-glycosidase activity. Alternatively, molecules disrupting intracellular trafficking have been shown to block ricin toxicity. Several research teams have recently developed high-throughput phenotypic screens for small molecules acting on the intracellular targets required for entry of ricin into cells. These screens have identified inhibitory compounds that can protect cells, and sometimes even animals against ricin. We review these newly discovered cellular inhibitors of ricin intoxication, discuss the advantages and drawbacks of chemical-genetics approaches, and address the issues to be resolved so that the therapeutic development of these small-molecule compounds can progress.
Keywords: chemical genetics; retrograde transport; ricin; small-molecule inhibitor.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

