Statistical Approach for Optimization of Physiochemical Requirements on Alkaline Protease Production from Bacillus licheniformis NCIM 2042
- PMID: 22347624
- PMCID: PMC3278927
- DOI: 10.1155/2012/905804
Statistical Approach for Optimization of Physiochemical Requirements on Alkaline Protease Production from Bacillus licheniformis NCIM 2042
Abstract
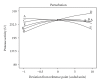
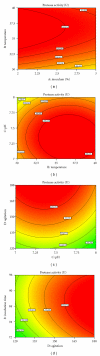
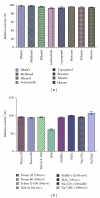
The optimization of physiochemical parameters for alkaline protease production using Bacillus licheniformis NCIM 2042 were carried out by Plackett-Burman design and response surface methodology (RSM). The model was validated experimentally and the maximum protease production was found 315.28 U using optimum culture conditions. The protease was purified using ammonium sulphate (60%) precipitation technique. The HPLC analysis of dialyzed sample showed that the retention time is 1.84 min with 73.5% purity. This enzyme retained more than 92% of its initial activity after preincubation for 30 min at 37°C in the presence of 25% v/v DMSO, methanol, ethanol, ACN, 2-propanol, benzene, toluene, and hexane. In addition, partially purified enzyme showed remarkable stability for 60 min at room temperature, in the presence of anionic detergent (Tween-80 and Triton X-100), surfactant (SDS), bleaching agent (sodium perborate and hydrogen peroxide), and anti-redeposition agents (Na(2)CMC, Na(2)CO(3)). Purified enzyme containing 10% w/v PEG 4000 showed better thermal, surfactant, and local detergent stability.
Figures



Similar articles
-
Purification and characterization of a thermo- and organic solvent-tolerant alkaline protease from Bacillus sp. JER02.Prep Biochem Biotechnol. 2015;45(2):128-43. doi: 10.1080/10826068.2014.907176. Prep Biochem Biotechnol. 2015. PMID: 24845261
-
Response-surface methodology for the production and the purification of a new H2 O2 -tolerant alkaline protease from Bacillus invictae AH1 strain.Biotechnol Prog. 2020 May;36(3):e2965. doi: 10.1002/btpr.2965. Epub 2020 Jan 30. Biotechnol Prog. 2020. PMID: 31951103
-
Stability of thermostable alkaline protease from Bacillus licheniformis RP1 in commercial solid laundry detergent formulations.Microbiol Res. 2008;163(3):299-306. doi: 10.1016/j.micres.2006.06.001. Epub 2006 Jul 26. Microbiol Res. 2008. PMID: 16872818
-
Purification and partial characterization of a detergent and oxidizing agent stable alkaline protease from a newly isolated Bacillus subtilis VSG-4 of tropical soil.J Microbiol. 2011 Jun;49(3):455-61. doi: 10.1007/s12275-011-0427-4. Epub 2011 Jun 30. J Microbiol. 2011. PMID: 21717332
-
Purification and properties of detergent-compatible extracellular alkaline protease from Scopulariopsis spp.Prep Biochem Biotechnol. 2014 Oct 3;44(7):738-59. doi: 10.1080/10826068.2013.854254. Prep Biochem Biotechnol. 2014. PMID: 24905049
Cited by
-
Proteomic analysis and optimized production of Alkalihalobacillus patagoniensis PAT 05T extracellular proteases.Bioprocess Biosyst Eng. 2021 Feb;44(2):225-234. doi: 10.1007/s00449-020-02436-z. Epub 2020 Sep 4. Bioprocess Biosyst Eng. 2021. PMID: 32888092
-
Production optimization of a heat-tolerant alkaline pectinase from Bacillus subtilis ZGL14 and its purification and characterization.Bioengineered. 2017 Sep 3;8(5):613-623. doi: 10.1080/21655979.2017.1292188. Epub 2017 Feb 16. Bioengineered. 2017. PMID: 28282260 Free PMC article.
-
Enhanced catalytic activity of Bacillus aryabhattai P1 protease by modulation with nanoactivator.Heliyon. 2020 Jun 4;6(6):e04053. doi: 10.1016/j.heliyon.2020.e04053. eCollection 2020 Jun. Heliyon. 2020. PMID: 32529068 Free PMC article.
-
Enhancement of feather degrading keratinase of Streptomyces swerraensis KN23, applying mutagenesis and statistical optimization to improve keratinase activity.BMC Microbiol. 2023 May 30;23(1):158. doi: 10.1186/s12866-023-02867-0. BMC Microbiol. 2023. PMID: 37248454 Free PMC article.
-
Novel Sequential Screening and Enhanced Production of Fibrinolytic Enzyme by Bacillus sp. IND12 Using Response Surface Methodology in Solid-State Fermentation.Biomed Res Int. 2017;2017:3909657. doi: 10.1155/2017/3909657. Epub 2017 Jan 17. Biomed Res Int. 2017. PMID: 28321408 Free PMC article.
References
-
- Shah K, Mody K, Keshri J, Jha B. Purification and characterization of a solvent, detergent and oxidizing agent tolerant protease from Bacillus cereus isolated from the Gulf of Khambhat. Journal of Molecular Catalysis B. 2010;67(1-2):85–91.
-
- Anwar A, Saleemuddin M. Alkaline protease from Spilosoma obliqua: potential applications in bio-formulations. Biotechnology and Applied Biochemistry. 2000;31(2):85–89. - PubMed
-
- Haki GD, Rakshit SK. Developments in industrially important thermostable enzymes: a review. Bioresource Technology. 2003;89(1):17–34. - PubMed
-
- Johnvesly B, Naik GR. Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochemistry. 2001;37(2):139–144.
-
- Fu Z, Hamid SBA, Razak CNA, Basri M, Bakar Salleh A, Rahman RNZA. Secretory expression in Escherichia coli and single-step purification of a heat-stable alkaline protease. Protein Expression and Purification. 2003;28(1):63–68. - PubMed
LinkOut - more resources
Full Text Sources

