Characterization of CdSe nanocrystals coated with amphiphiles. A capillary electrophoresis study
- PMID: 22347727
- PMCID: PMC3267930
- DOI: 10.1007/s00604-011-0727-8
Characterization of CdSe nanocrystals coated with amphiphiles. A capillary electrophoresis study
Abstract
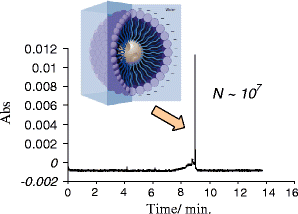
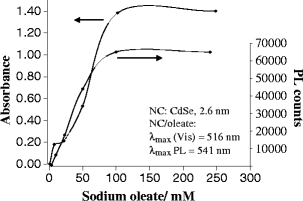
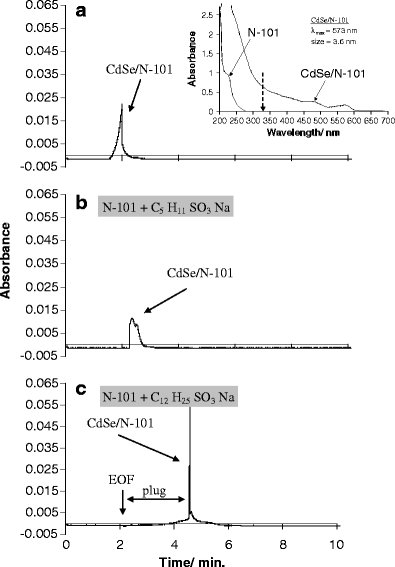
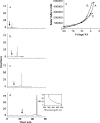
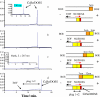
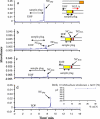
We have synthesized CdSe nanocrystals (NCs) possessing a trioctylphosphine surface passivation layer and modified with amphiphilic molecules to form a surface bilayer. The NCs covered with single amphiphiles are not stable in aqueous solution, but a mixed amphiphilic system is shown to provide stability in solution over several months. The solutions of the modified NCs were characterized by UV-Vis absorbance, photoluminescence, and transmission electron microscopy. An electrophoretic study revealed two operational modes. The first relies on the enrichment of NCs using a micellar plug as a tool. The accumulation of NCs at the plug-electrolyte buffer interface results in a sharp peak. By controlling the electrophoretic conditions, nanocrystals were forced to exit a micellar plug into an electrolyte buffer. We conclude that a system consisting of modified nanocrystals and a micellar plug can act as a mixed pseudomicellar system, where modified nanocrystals play the role of pseudomicelles.FigureElectrophoretic focusing of amphiphile coated CdSe nanocrystals using a micellar plug. ELECTRONIC SUPPLEMENTARY MATERIAL: The online version of this article (doi:10.1007/s00604-011-0727-8) contains supplementary material, which is available to authorized users.
Figures

References
-
- Yu WW, Qu L, Guo W, Peng X. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater. 2003;15:2854–2860. doi: 10.1021/cm034081k. - DOI
-
- Sergeev GB. Nanochemistry. Amsterdam: Elsevier B.V; 2006.
LinkOut - more resources
Full Text Sources
