Protonation of Homoenolate Equivalents Generated by N-Heterocyclic Carbenes
- PMID: 22347730
- PMCID: PMC3279924
- DOI: 10.1055/s-2008-1072516
Protonation of Homoenolate Equivalents Generated by N-Heterocyclic Carbenes
Abstract
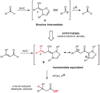
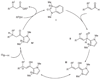
Homoenolate equivalents are generated by Lewis basic N-heterocyclic carbene catalysts and then protonated to generate efficiently saturated esters from unsaturated aldehydes. This reactivity is extended to the generation of β-acylvinyl anions from alkynyl aldehydes. The asymmetric protonation of a homoenolate equivalent generated from a β,β-disubstituted aldehyde can be accomplished with a chiral N-heterocyclic carbene.
Figures
Similar articles
-
Conversion of alpha,beta-unsaturated aldehydes into saturated esters: an Umpolung reaction catalyzed by nucleophilic carbenes.Org Lett. 2005 Mar 3;7(5):905-8. doi: 10.1021/ol050100f. Org Lett. 2005. PMID: 15727471
-
Chiral N-heterocyclic carbene-catalyzed generation of ester enolate equivalents from {alpha},{beta}-unsaturated aldehydes for enantioselective Diels-Alder reactions.Proc Natl Acad Sci U S A. 2010 Nov 30;107(48):20661-5. doi: 10.1073/pnas.1007469107. Epub 2010 Oct 25. Proc Natl Acad Sci U S A. 2010. PMID: 20974930 Free PMC article.
-
Enantio- and Diastereoselective Hydrofluorination of Enals by N-Heterocyclic Carbene Catalysis.Angew Chem Int Ed Engl. 2019 May 27;58(22):7410-7414. doi: 10.1002/anie.201902989. Epub 2019 Apr 25. Angew Chem Int Ed Engl. 2019. PMID: 30942950
-
N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Remote Enantioselective Functionalizations.Angew Chem Int Ed Engl. 2018 Apr 3;57(15):3862-3873. doi: 10.1002/anie.201709684. Epub 2018 Mar 9. Angew Chem Int Ed Engl. 2018. PMID: 29136320 Review.
-
A Review on Generation and Reactivity of the N-Heterocyclic Carbene-Bound Alkynyl Acyl Azolium Intermediates.Molecules. 2022 Nov 17;27(22):7990. doi: 10.3390/molecules27227990. Molecules. 2022. PMID: 36432089 Free PMC article. Review.
Cited by
-
Exploiting Acyl and Enol Azolium Intermediates via NHeterocyclic Carbene Catalyzed Reactions of Alpha-Reducible Aldehydes.Adv Synth Catal. 2012 Jun 18;354(9):1617-1639. doi: 10.1002/adsc.201200031. Epub 2012 Apr 19. Adv Synth Catal. 2012. PMID: 23538785 Free PMC article.
-
Self-Supported N-Heterocyclic Carbenes and Their Use as Organocatalysts.Molecules. 2016 Aug 20;21(8):1100. doi: 10.3390/molecules21081100. Molecules. 2016. PMID: 27556435 Free PMC article.
-
Enantioselective SN 2 Alkylation of Homoenolates by N-Heterocyclic Carbene Catalysis.Adv Sci (Weinh). 2023 Oct;10(29):e2303517. doi: 10.1002/advs.202303517. Epub 2023 Aug 4. Adv Sci (Weinh). 2023. PMID: 37541670 Free PMC article.
-
Radical coupling of β-ketoesters and amides promoted by Brønsted/Lewis acids.Green Synth Catal. 2020 Jun;1(1):70-74. doi: 10.1016/j.gresc.2020.06.003. Epub 2020 Jul 27. Green Synth Catal. 2020. PMID: 34485961 Free PMC article.
-
Enantioselective protonation.Nat Chem. 2009 Aug;1(5):359-69. doi: 10.1038/nchem.297. Nat Chem. 2009. PMID: 20428461 Free PMC article. Review.
References
-
- Seebach D. Angew. Chem., Int. Ed. Engl. 1979;18:239.
-
- Eisch JJ. J. Organomet. Chem. 1995;500:101.
-
- Pohl M, Lingen B, Muller M. Chem. Eur. J. 2002;8:5289. - PubMed
-
- Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606. - PubMed
-
- Marion N, Diez-Gonzalez S, Nolan IP. Angew. Chem. Int. Ed. 2007;46:2988. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources