The spin label amino acid TOAC and its uses in studies of peptides: chemical, physicochemical, spectroscopic, and conformational aspects
- PMID: 22347893
- PMCID: PMC3271205
- DOI: 10.1007/s12551-011-0064-5
The spin label amino acid TOAC and its uses in studies of peptides: chemical, physicochemical, spectroscopic, and conformational aspects
Abstract
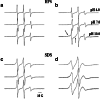
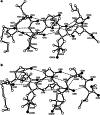
We review work on the paramagnetic amino acid 2,2,6,6-tetramethyl-N-oxyl-4-amino-4-carboxylic acid, TOAC, and its applications in studies of peptides and peptide synthesis. TOAC was the first spin label probe incorporated in peptides by means of a peptide bond. In view of the rigid character of this cyclic molecule and its attachment to the peptide backbone via a peptide bond, TOAC incorporation has been very useful to analyze backbone dynamics and peptide secondary structure. Many of these studies were performed making use of EPR spectroscopy, but other physical techniques, such as X-ray crystallography, CD, fluorescence, NMR, and FT-IR, have been employed. The use of double-labeled synthetic peptides has allowed the investigation of their secondary structure. A large number of studies have focused on the interaction of peptides, both synthetic and biologically active, with membranes. In the latter case, work has been reported on ligands and fragments of GPCR, host defense peptides, phospholamban, and β-amyloid. EPR studies of macroscopically aligned samples have provided information on the orientation of peptides in membranes. More recent studies have focused on peptide-protein and peptide-nucleic acid interactions. Moreover, TOAC has been shown to be a valuable probe for paramagnetic relaxation enhancement NMR studies of the interaction of labeled peptides with proteins. The growth of the number of TOAC-related publications suggests that this unnatural amino acid will find increasing applications in the future.
Figures



Similar articles
-
Backbone dynamics determined by electron paramagnetic resonance to optimize solid-phase peptide synthesis of TOAC-labeled phospholamban.Biopolymers. 2007;88(1):29-35. doi: 10.1002/bip.20618. Biopolymers. 2007. PMID: 17066471
-
Tracking amyloid oligomerization with monomer resolution using a 13-amino acid peptide with a backbone-fixed spin label.Phys Chem Chem Phys. 2019 Dec 7;21(45):25187-25195. doi: 10.1039/c9cp01060b. Epub 2019 Nov 7. Phys Chem Chem Phys. 2019. PMID: 31696167
-
Mobility of TOAC spin-labelled peptides binding to the Src SH3 domain studied by paramagnetic NMR.J Biomol NMR. 2008 Jul;41(3):157-67. doi: 10.1007/s10858-008-9248-0. Epub 2008 Jun 17. J Biomol NMR. 2008. PMID: 18560762 Free PMC article.
-
TOAC spin labels in the backbone of alamethicin: EPR studies in lipid membranes.Biophys J. 2007 Jan 15;92(2):473-81. doi: 10.1529/biophysj.106.092775. Epub 2006 Oct 20. Biophys J. 2007. PMID: 17056731 Free PMC article.
-
Spectroscopic and structural elucidation of amino acid derivatives and small peptides: experimental and theoretical tools.Amino Acids. 2010 Jan;38(1):45-50. doi: 10.1007/s00726-008-0220-9. Epub 2008 Dec 14. Amino Acids. 2010. PMID: 19083080 Review.
Cited by
-
Enzymatic Spin-Labeling of Protein N- and C-Termini for Electron Paramagnetic Resonance Spectroscopy.Bioconjug Chem. 2023 Mar 15:10.1021/acs.bioconjchem.3c00029. doi: 10.1021/acs.bioconjchem.3c00029. Online ahead of print. Bioconjug Chem. 2023. PMID: 36921260 Free PMC article.
-
Trajectory-Based Simulation of EPR Spectra: Models of Rotational Motion for Spin Labels on Proteins.J Phys Chem B. 2019 Dec 5;123(48):10131-10141. doi: 10.1021/acs.jpcb.9b02693. Epub 2019 Nov 21. J Phys Chem B. 2019. PMID: 31693365 Free PMC article.
-
Pulsed EPR distance measurements in soluble proteins by site-directed spin labeling (SDSL).Curr Protoc Protein Sci. 2013 Nov 5;74:17.17.1-17.17.29. doi: 10.1002/0471140864.ps1717s74. Curr Protoc Protein Sci. 2013. PMID: 24510645 Free PMC article.
-
Light-Emitting Probes for Labeling Peptides.Cell Rep Phys Sci. 2020 Dec 23;1(12):100257. doi: 10.1016/j.xcrp.2020.100257. Epub 2020 Nov 25. Cell Rep Phys Sci. 2020. PMID: 34396352 Free PMC article.
-
Electron Paramagnetic Resonance as a Tool for Studying Membrane Proteins.Biomolecules. 2020 May 13;10(5):763. doi: 10.3390/biom10050763. Biomolecules. 2020. PMID: 32414134 Free PMC article. Review.
References
-
- Altenbach C, Flitsch SL, Khorana HG, Hubbell WL. Structural studies on transmembrane proteins. 2. Spin labeling of bacteriorhodopsin mutants at unique cysteines. Biochemistry. 1989;28:7806–7812. - PubMed
-
- Anderson DJ, Hanson P, McNulty J, Millhauser G, Monaco V, Formaggio F, Crisma M, Toniolo C. Solution structures of TOAC-labeled trichogin GA IV peptides from allowed (g ≈ 2) and half-field electron spin resonance. J Am Chem Soc. 1999;121:6919–6927.
-
- Atherton E, Sheppard RC. Solid phase peptide synthesis: a practical approach. Oxford: I.L.R. Press; 1989.
-
- Balog M, Kálai T, Jeko J, Berente Z, Steinhoff H-J, Engelhard M, Hideg K. Synthesis of new conformationally rigid paramagnetic α-amino acids. Tetrahedron Lett. 2003;44:9213–9217.
-
- Barbosa SR, Cilli EM, Lamy-Freund MT, Castrucci AM, Nakaie CR. First synthesis of a fully active spin-labeled peptide hormone. FEBS Lett. 1999;446:45–48. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

