HIF-independent regulation of thioredoxin reductase 1 contributes to the high levels of reactive oxygen species induced by hypoxia
- PMID: 22348009
- PMCID: PMC3278416
- DOI: 10.1371/journal.pone.0030470
HIF-independent regulation of thioredoxin reductase 1 contributes to the high levels of reactive oxygen species induced by hypoxia
Abstract
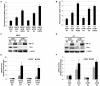
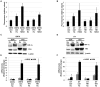
Cellular adaptation to hypoxic conditions mainly involves transcriptional changes in which hypoxia inducible factors (HIFs) play a critical role. Under hypoxic conditions, HIF protein is stabilized due to inhibition of the activity of prolyl hydroxylases (EGLNs). Because the reaction carried out by these enzymes uses oxygen as a co-substrate it is generally accepted that the hypoxic inhibition of EGLNs is due to the reduction in oxygen levels. However, several studies have reported that hypoxic generation of mitochondrial reactive oxygen species (ROS) is required for HIF stabilization. Here, we show that hypoxia downregulates thioredoxin reductase 1 (TR1) mRNA and protein levels. This hypoxic TR1 regulation is HIF independent, as HIF stabilization by EGLNs inhibitors does not affect TR1 expression and HIF deficiency does not block TR1 hypoxic-regulation, and it has an effect on TR1 function, as hypoxic conditions also reduce TR1 activity. We found that, when cultured under hypoxic conditions, TR1 deficient cells showed a larger accumulation of ROS compared to control cells, whereas TR1 over-expression was able to block the hypoxic generation of ROS. Furthermore, the changes in ROS levels observed in TR1 deficient or TR1 over-expressing cells did not affect HIF stabilization or function. These results indicate that hypoxic TR1 down-regulation is important in maintaining high levels of ROS under hypoxic conditions and that HIF stabilization and activity do not require hypoxic generation of ROS.
Conflict of interest statement
Figures




Similar articles
-
Regulation of HIF-1α activity by overexpression of thioredoxin is independent of thioredoxin reductase status.Mol Cells. 2013 Aug;36(2):151-7. doi: 10.1007/s10059-013-0121-y. Epub 2013 Aug 1. Mol Cells. 2013. PMID: 23912593 Free PMC article.
-
Hypoxic stabilization of mRNA is HIF-independent but requires mtROS.Cell Mol Biol Lett. 2018 Oct 4;23:48. doi: 10.1186/s11658-018-0112-2. eCollection 2018. Cell Mol Biol Lett. 2018. PMID: 30305827 Free PMC article.
-
Stabilization of hypoxia-inducible factor-1alpha protein in hypoxia occurs independently of mitochondrial reactive oxygen species production.J Biol Chem. 2010 Oct 8;285(41):31277-84. doi: 10.1074/jbc.M110.158485. Epub 2010 Jul 30. J Biol Chem. 2010. PMID: 20675386 Free PMC article.
-
Mitochondrial reactive oxygen species are required for hypoxic HIF alpha stabilization.Adv Exp Med Biol. 2006;588:165-70. doi: 10.1007/978-0-387-34817-9_15. Adv Exp Med Biol. 2006. PMID: 17089888 Review.
-
[The new face of factors induced by hypoxia--HIF-1 and HIF-2 and oxidative stress].Postepy Biochem. 2010;56(2):156-64. Postepy Biochem. 2010. PMID: 20873110 Review. Polish.
Cited by
-
Hypoxia-Induced Changes in L-Cysteine Metabolism and Antioxidative Processes in Melanoma Cells.Biomolecules. 2023 Oct 7;13(10):1491. doi: 10.3390/biom13101491. Biomolecules. 2023. PMID: 37892173 Free PMC article.
-
Hypoxia Signaling in Parkinson's Disease: There Is Use in Asking "What HIF?".Biology (Basel). 2021 Jul 29;10(8):723. doi: 10.3390/biology10080723. Biology (Basel). 2021. PMID: 34439955 Free PMC article. Review.
-
Prolyl hydroxylase domain enzymes: important regulators of cancer metabolism.Hypoxia (Auckl). 2014 Aug 30;2:127-142. doi: 10.2147/HP.S47968. eCollection 2014. Hypoxia (Auckl). 2014. PMID: 27774472 Free PMC article. Review.
-
Tumour Microenvironment Stress Promotes the Development of Drug Resistance.Antioxidants (Basel). 2021 Nov 11;10(11):1801. doi: 10.3390/antiox10111801. Antioxidants (Basel). 2021. PMID: 34829672 Free PMC article. Review.
-
Hydrogen peroxide sensing, signaling and regulation of transcription factors.Redox Biol. 2014 Feb 23;2:535-62. doi: 10.1016/j.redox.2014.02.006. eCollection 2014. Redox Biol. 2014. PMID: 24634836 Free PMC article. Review.
References
-
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. - PubMed
-
- Seta KA, Spicer Z, Yuan Y, Lu G, Millhorn DE. Responding to hypoxia: lessons from a model cell line. Sci STKE. 2002;146:RE11. - PubMed
-
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. - PubMed
-
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. - PubMed
-
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

